Pathologic responses to neoadjuvant chemoimmunotherapy in primary limited-stage small-cell lung cancer
- PMID: 36208136
- PMCID: PMC9663685
- DOI: 10.1111/1759-7714.14679
Pathologic responses to neoadjuvant chemoimmunotherapy in primary limited-stage small-cell lung cancer
Abstract
Background: Immunotherapy has been proved to have a large effect on extensive-stage small cell lung cancer, but the role of immunotherapy in limited-stage small-cell lung cancer (LS-SCLC) is still unknown.
Methods: A retrospective study of six patients with LS-SCLC who were treated with neoadjuvant chemoimmunotherapy (durvalumab plus etoposide combined with cisplatin) was performed. Patients were evaluated by the safety, feasibility and pathologic responses of neoadjuvant chemoimmunotherapy.
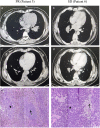
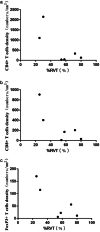
Results: Neoadjuvant durvalumab combined chemotherapy was associated with few immediate adverse events and did not delay planned surgery. All patients achieved partial pathologic response (pPR) instead of major pathologic response, or pathologic complete response. No association was observed between programmed death-ligand 1 expression in tumor specimens and the pathologic response. However, tumors with high expression of immune cells such as CD4+ T cells, CD8+ T cells and FoxP3+ Tregs tended to have better pathologic responses than tumors with low expression of immune cells.
Conclusions: Neoadjuvant durvalumab combined chemotherapy could induce pPR with few side effects in resectable LS-SCLC. The immune cells in the tumor microenvironment might play an important role in neoadjuvant chemoimmunotherapy in resectable LS-SCLC.
Keywords: PD-L1; limited-stage small-cell lung cancer (LS-SCLC); neoadjuvant chemoimmunotherapy; partial pathologic response; surgery.
© 2022 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. - PubMed
-
- Abdelhamid K, Kakourou A, Degrauwe N, Nikolopoulou A, Bouchaab H, Peters S, et al. Small‐cell lung cancer: management and novelties. Rev Med Suisse. 2020;16(695):1079–85. - PubMed
-
- Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small‐cell lung cancer using the National Cancer Data Base. J Thorac Oncol. 2015;10(2):316–23. - PubMed
-
- Calles A, Aguado G, Sandoval C, Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961–76. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

