Design and evaluation of novel analogs of 2-amino-4-boronobutanoic acid (ABBA) as inhibitors of human gamma-glutamyl transpeptidase
- PMID: 36208545
- PMCID: PMC10340724
- DOI: 10.1016/j.bmc.2022.116986
Design and evaluation of novel analogs of 2-amino-4-boronobutanoic acid (ABBA) as inhibitors of human gamma-glutamyl transpeptidase
Abstract
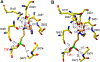
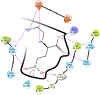
Inhibitors of gamma-glutamyl transpeptidase (GGT1, aka gamma-glutamyl transferase) are needed for the treatment of cancer, cardiovascular illness and other diseases. Compounds that inhibit GGT1 have been evaluated in the clinic, but no inhibitor has successfully demonstrated specific and systemic GGT1 inhibition. All have severe side effects. L-2-amino-4‑boronobutanoic acid (l-ABBA), a glutamate analog, is the most potent GGT1 inhibitor in vitro. In this study, we have solved the crystal structure of human GGT1 (hGGT1) with ABBA bound in the active site. The structure was interrogated to identify interactions between the enzyme and the inhibitor. Based on these data, a series of novel ABBA analogs were designed and synthesized. Their inhibitory activity against the hydrolysis and transpeptidation activities of hGGT1 were determined. The lead compounds were crystalized with hGGT1 and the structures solved. The kinetic data and structures of the complexes provide new insights into the critical role of protein structure dynamics in developing compounds for inhibition of hGGT1.
Keywords: Computer modeling; Drug development; Enzyme inhibitor; Enzyme structure; Gamma-glutamyl transferase; Gamma-glutamyl transpeptidase; Molecular docking; Molecular modeling; Protein crystallization; Structure-based drug design.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

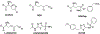
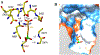

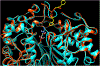
References
-
- Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. - PubMed
-
- Hanigan HM. Gamma-Glutamyl Transferases. In: Jez J, ed. Encyclopedia of Biological Chemistry. 3rd Edition. Oxford: Elsevier Press; 2021:43–50.
-
- Hanigan MH, Frierson HF Jr. Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem: Off J Histochem Soc. 1996;44:1101–1108. - PubMed
-
- Hanigan MH, Frierson HF, Swanson PE, De Young BR. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum Pathol. 1999;30:300–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

