A randomized phase 2 clinical trial of phentolamine mesylate eye drops in patients with severe night vision disturbances
- PMID: 36209072
- PMCID: PMC9548101
- DOI: 10.1186/s12886-022-02621-6
A randomized phase 2 clinical trial of phentolamine mesylate eye drops in patients with severe night vision disturbances
Abstract
Purpose: Dim light vision disturbances (DLD) comprise a wide range of symptoms affecting the quality of vision at low illumination including glare, halos, and starbursts. This exploratory study investigated 1.0% phentolamine mesylate ophthalmic solution (PMOS) as a treatment to improve vision and image quality for patients with DLD.
Methods: In this placebo-controlled, randomized, double-masked clinical trial, 24 adult patients with severe DLD were randomized in a 2:1 ratio to receive either one dose of PMOS or placebo. Subjects were eligible if they reported experiencing severe night vision difficulty that was not eliminated by distance spectacle correction and scored ≥0.3 log units below the normal range of contrast sensitivity assessed under mesopic conditions with glare at ≥2 spatial frequencies. Key efficacy outcomes were change from baseline in pupil diameter, contrast sensitivity, and visual acuity. Safety measures including intraocular pressure, conjunctival hyperemia, and systemic effects were also assessed.
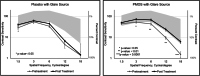
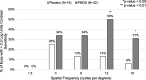
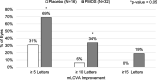
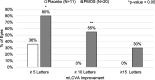
Results: Eight subjects were randomized to placebo (63% female; mean age 47 years) and 16 were randomized to PMOS (75% female; mean age 42 years). Mean (SD) pupil diameter of PMOS-treated subjects decreased significantly - 1.3 mm (0 to - 2.8 mm) with p < 0.0001. Mean contrast sensitivity with glare in PMOS-treated subjects improved significantly post-treatment at spatial frequencies 3, 6, 12, and 18 cycles per degree (p ≤ 0.03). PMOS also demonstrated improvements in the numbers of letters read for mesopic and photopic, high- and low-contrast visual acuity (LCVA). Importantly, a statistically greater proportion of PMOS-treated eyes registered mesopic LCVA 5 letter (69% vs. 31%, p = 0.029) and 10 letter (34% vs. 6%, p = 0.04) improvement, with a trend at 15 letters (19% vs. 0%, p = 0.16). PMOS was well tolerated with the only reported side effect being a mild increase in conjunctival hyperemia.
Conclusion: PMOS was well tolerated and effectively reduced pupil size with improvements in contrast sensitivity and visual acuity in adults with severe DLD. Future Phase 3 studies should be conducted to further evaluate its potential to treat DLD.
Trial registration: The trial registration number is NCT04004507 (02/07/2019). Retrospectively registered.
Keywords: DLD; Dim Light Disturbance; Glare; Halos; NVD; Night Vision Disturbance; Phentolamine; Photic Phenomenon; Starburst.
© 2022. The Author(s).
Conflict of interest statement
JSP, MB, CH, JY, KR, AK, MH, CM, AL, KC, and MS are or were employees, directors/officers, or consultants for Ocuphire Pharma. JP, EL, and MM are on the medical advisory board for Ocuphire Pharma. MM was the principal investigator for this clinical trial funded by Ocularis Pharma (now Ocuphire Pharma). JSP reports personal fees from Acufocus, Kala Pharmaceuticals, Keeler, MG Therapeutics, Mimetogen Pharmaceuticals, Novartis, Ocunexis Therapeutics, Okogen, Stuart Pharmaceuticals, Sun Pharma, Thea Pharma, TearLab, and Ocuphire. MM reports personal fees from Allergan, Alcon, Bausch and Lomb, Eyevance, Novartis, Tarsus, Visus, Aperta, Ocusoft, OCULUS USA, DOMPE, BioTissue, BlephEx, Akorn, Quidel, ORCA Surgical, TearLab, J&J Vision, TearCare, NuLids, Ocuphire, STROMA, Hellas LTD, Sun Pharma, Avedro, Omeros, Scope, and Sight Sciences. The authors indicated that they have no other conflicts of interest with regard to the content of this article.
Figures


References
-
- Bidgoli S, Alio JL. Night Vision Disturbances Following Refractive Surgery: Causes, Prevention, and Treatment. In: Alio JL, Azar DT, editors. Management of Complications in Refractive Surgery. Cham: Springer International Publishing; 2018. pp. 163–174.
-
- Alio JL, Azar DT. Management of complications in refractive surgery. Springer; 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

