Identifying optimal vaccination scenarios to reduce varicella zoster virus transmission and reactivation
- PMID: 36209074
- PMCID: PMC9548166
- DOI: 10.1186/s12916-022-02534-7
Identifying optimal vaccination scenarios to reduce varicella zoster virus transmission and reactivation
Abstract
Background: Varicella zoster virus (VZV) is one of the eight known human herpesviruses. Initial VZV infection results in chickenpox, while viral reactivation following a period of latency manifests as shingles. Separate vaccines exist to protect against both initial infection and subsequent reactivation. Controversy regarding chickenpox vaccination is contentious with most countries not including the vaccine in their childhood immunization schedule due to the hypothesized negative impact on immune-boosting, where VZV reactivation is suppressed through exogenous boosting of VZV antibodies from exposure to natural chickenpox infections.
Methods: Population-level chickenpox and shingles notifications from Thailand, a country that does not vaccinate against either disease, were previously fitted with mathematical models to estimate rates of VZV transmission and reactivation. Here, multiple chickenpox and shingles vaccination scenarios were simulated and compared to a model lacking any vaccination to analyze the long-term impacts of VZV vaccination.
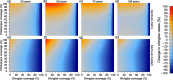
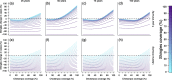
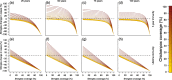
Results: As expected, simulations suggested that an introduction of the chickenpox vaccine, at any coverage level, would reduce chickenpox incidence. However, chickenpox vaccine coverage levels above 35% would increase shingles incidence under realistic estimates of shingles coverage with the current length of protective immunity from the vaccine. A trade-off between chickenpox and shingles vaccination coverage was discovered, where mid-level chickenpox coverage levels were identified as the optimal target to minimize total zoster burden. Only in scenarios where shingles vaccine provided lifelong immunity or coverage exceeded current levels could large reductions in both chickenpox and shingles be achieved.
Conclusions: The complicated nature of VZV makes it impossible to select a single vaccination scenario as universal policy. Strategies focused on reducing both chickenpox and shingles incidence, but prioritizing the latter should maximize efforts towards shingles vaccination, while slowly incorporating chickenpox vaccination. Alternatively, countries may wish to minimize VZV complications of both chickenpox and shingles, which would lead to maximizing vaccine coverage levels across both diseases. Balancing the consequences of vaccination to overall health impacts, including understanding the impact of an altered mean age of infection for both chickenpox and shingles, would need to be considered prior to any vaccine introduction.
Keywords: Chickenpox; Mathematical modeling; Shingles; Vaccination; Varicella zoster virus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis. 2016;35(5):723–34. - PubMed
-
- Wells MW. The Seasonal Patterns of Measles and Chicken Pox. Am J Hyg. 1944;40(3):279–317.
-
- Sengupta N, Brewer J. A global perspective of the epidemiology and burden of varicella-zoster virus. Curr Pediatr Rev. 2009;5(4):207–28. doi: 10.2174/157339609791317315. - DOI
-
- WHO. Background Paper on Varicella Vaccine: SAGE Working Group on Varicella and Herpes Zoster Vaccines. 2014. http://www.who.int/immunization/sage/meetings/2014/april/1_SAGE_varicell.... Accessed 15 Sept 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

