The emerging role of pyroptosis in pediatric cancers: from mechanism to therapy
- PMID: 36209102
- PMCID: PMC9547461
- DOI: 10.1186/s13045-022-01365-6
The emerging role of pyroptosis in pediatric cancers: from mechanism to therapy
Abstract
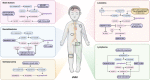
Pediatric cancers are the driving cause of death for children and adolescents. Due to safety requirements and considerations, treatment strategies and drugs for pediatric cancers have been so far scarcely studied. It is well known that tumor cells tend to progressively evade cell death pathways, which is known as apoptosis resistance, one of the hallmarks of cancer, dominating tumor drug resistance. Recently, treatments targeting nonapoptotic cell death have drawn great attention. Pyroptosis, a newly specialized form of cell death, acts as a critical physiological regulator in inflammatory reaction, cell development, tissue homeostasis and stress response. The action in different forms of pyroptosis is of great significance in the therapy of pediatric cancers. Pyroptosis could be induced and consequently modulate tumorigenesis, progression, and metastasis if treated with local or systemic therapies. However, excessive or uncontrolled cell death might lead to tissue damage, acute inflammation, or even cytokine release syndrome, which facilitates tumor progression or recurrence. Herein, we aimed to describe the molecular mechanisms of pyroptosis, to highlight and discuss the challenges and opportunities for activating pyroptosis pathways through various oncologic therapies in multiple pediatric neoplasms, including osteosarcoma, neuroblastoma, leukemia, lymphoma, and brain tumors.
Keywords: Cytokine release syndrome; Osteosarcoma; Pediatric cancer; Programmed cell death; Pyroptosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
- 2021Szvup169/the Science and Technology Development Fund Guided by Central Government
- 82272664/National Natural Science Foundation of China
- 2022JJ30843/Hunan Provincial Natural Science Foundation of China
- D2022117/Hunan Provincial Administration of Traditional Chinese Medicine Project
- 81902745/National Natural Foundation of China
LinkOut - more resources
Full Text Sources
Medical

