The journey of CAR-T therapy in hematological malignancies
- PMID: 36209106
- PMCID: PMC9547409
- DOI: 10.1186/s12943-022-01663-0
The journey of CAR-T therapy in hematological malignancies
Abstract
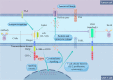
Chimeric antigen receptor T (CAR-T) cells therapy has revolutionized the treatment paradigms for hematological malignancies, with multi-line therapy-refractory patients achieving durable complete remissions (CR) and relatively high objective response rate (ORR). So far, many CAR-T products, such as Kymriah, Yescarta and Tecartus, have been developed and got the unprecedented results. However, some patients may relapse afterwards, driving intense investigations into promoting the development of novel strategies to overcome resistance and mechanisms of relapse. Notable technical progress, such as nanobodies and CRISPR-Case9, has also taken place to ensure CAR-T cell therapy fully satisfies its medical potential. In this review, we outline the basic principles for the development and manufacturing processes of CAR-T cell therapy, summarize the similarities and differences in efficacy of different products as well as their corresponding clinical results, and discuss CAR-T immunotherapy combined with other clinical effects of drug therapy.
Keywords: CAR-T cell therapy; Combinatorial therapy; Drug product; Hematological malignancies; Targeted therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Flinn IW, Jaeger U, Shah NN, Blaise D, Briones J, Shune L, et al. A first-in-human study of YTB323, a novel, autologous CD19-directed CAR-T cell therapy manufactured using the novel T-charge TM platform, for the treatment of patients (pts) with relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) Blood. 2021;138:740. doi: 10.1182/blood-2021-146268. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

