Real-world characteristics, modern antidiabetic treatment patterns, and comorbidities of patients with type 2 diabetes in central and Eastern Europe: retrospective cross-sectional and longitudinal evaluations in the CORDIALLY® study
- PMID: 36209118
- PMCID: PMC9548172
- DOI: 10.1186/s12933-022-01631-4
Real-world characteristics, modern antidiabetic treatment patterns, and comorbidities of patients with type 2 diabetes in central and Eastern Europe: retrospective cross-sectional and longitudinal evaluations in the CORDIALLY® study
Abstract
Background: Guidelines from 2016 onwards recommend early use of SGLT2i or GLP-1 RA for patients with type 2 diabetes (T2D) and cardiovascular disease (CVD), to reduce CV events and mortality. Many eligible patients are not treated accordingly, although data are lacking for Central and Eastern Europe (CEE).
Methods: The CORDIALLY non-interventional study evaluated the real-world characteristics, modern antidiabetic treatment patterns, and the prevalence of CVD and chronic kidney disease (CKD) in adults with T2D at nonhospital-based practices in CEE. Data were retrospectively collated by medical chart review for patients initiating empagliflozin, another SGLT2i, DPP4i, or GLP-1 RA in autumn 2018. All data were analysed cross-sectionally, except for discontinuations assessed 1 year ± 2 months after initiation.
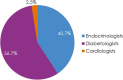
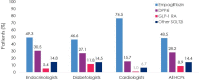
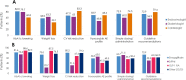
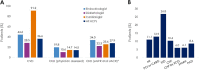
Results: Patients (N = 4055) were enrolled by diabetologists (56.7%), endocrinologists (40.7%), or cardiologists (2.5%). Empagliflozin (48.5%) was the most prescribed medication among SGLT2i, DPP4i, and GLP-1 RA; > 3 times more patients were prescribed empagliflozin than other SGLT2i (10 times more by cardiologists). Overall, 36.6% of patients had diagnosed CVD. Despite guidelines recommending SGLT2i or GLP-1 RA, 26.8% of patients with CVD received DPP4i. Patients initiating DPP4i were older (mean 66.4 years) than with SGLT2i (62.4 years) or GLP-1 RA (58.3 years). CKD prevalence differed by physician assessment (14.5%) or based on eGFR and UACR (27.9%). Many patients with CKD (≥ 41%) received DPP4i, despite guidelines recommending SGLT2is owing to their renal benefits. 1 year ± 2-months after initiation, 10.0% (7.9-12.3%) of patients had discontinued study medication: 23.7-45.0% due to 'financial burden of co-payment', 0-1.9% due to adverse events (no patients discontinued DPP4i due to adverse events). Treatment guidelines were 'highly relevant' for a greater proportion of cardiologists (79.4%) and endocrinologists (72.9%) than diabetologists (56.9%), and ≤ 20% of physicians consulted other physicians when choosing and discontinuing treatments.
Conclusions: In CORDIALLY, significant proportions of patients with T2D and CVD/CKD who initiated modern antidiabetic medication in CEE in autumn 2018 were not treated with cardioprotective T2D medications. Use of DPP4i instead of SGLT2i or GLP-1 RA may be related to lack of affordable access, the perceived safety of these medications, lack of adherence to the latest treatment guidelines, and lack of collaboration between physicians. Thus, many patients with T2D and comorbidities may develop preventable complications or die prematurely. Trial registration NCT03807440.
Keywords: Cardiovascular disease (CVD); Cardiovascular outcomes trials (CVOTs); Cardiovascular safety; Chronic kidney disease (CKD); Dipeptidyl peptidase-4 inhibitors (DPP4i); Glucagon-like peptide-1 receptor agonists (GLP-1 RA); Glucose-lowering drug; Sodium-glucose cotransporter-2 inhibitors (SGLT2i); Type 2 diabetes.
© 2022. The Author(s).
Conflict of interest statement
Martin Prázný has received speaker honoraria and has consulted for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Novo Nordisk, Medtronic, MSD, Mundipharma, Sanofi, and Teva. Lyudmila Suplotova declares associations (member of advisory board, lecturer, clinical trial investigator) with the following companies: Boehringer Ingelheim, AstraZeneca, Berlin Chemie, Merck, MSD, Novartis, Novo Nordisk, STADA. Janusz Gumprecht has received fees from Abbott Diabetes Care for lecturing and participating in the advisory panels. Zdravko Kamenov declares associations (member of advisory board, lecturer, clinical trial investigator) with the following companies: Actavis, AstraZeneca, Bayer Schering, Berlin Chemie, Boehringer Ingelheim, Elli Lily, LoLi pharma, Merck, MSD, Mundi pharma, Mylan, Novartis, Novo Nordisk, Pfizer, Sandoz, Sanofi, and Servier. Tibor Fülöp reports no conflicts of interest. Alexey Medvedchikov, Doron Rosenzweig, and Milos Aleksandric are employees of Boehringer Ingelheim.
Figures

Similar articles
-
SGLT2i and GLP-1 RA therapy in type 1 diabetes and reno-vascular outcomes: a real-world study.Diabetologia. 2023 Oct;66(10):1869-1881. doi: 10.1007/s00125-023-05975-8. Epub 2023 Jul 28. Diabetologia. 2023. PMID: 37505282 Free PMC article.
-
Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease.Cardiovasc Diabetol. 2022 Nov 5;21(1):232. doi: 10.1186/s12933-022-01676-5. Cardiovasc Diabetol. 2022. PMID: 36335326 Free PMC article.
-
Patient and physician factors driving the gaps in use of drugs with cardiovascular and kidney benefits by medicare beneficiaries with type 2 diabetes treated by endocrinologists, nephrologists, and cardiologists: Population-based cohort study.Diabetes Res Clin Pract. 2025 Mar;221:112039. doi: 10.1016/j.diabres.2025.112039. Epub 2025 Feb 7. Diabetes Res Clin Pract. 2025. PMID: 39923965
-
Effects of new hypoglycemic drugs on patients with heart failure: a systematic review and network meta-analysis.Postgrad Med J. 2025 Mar 16;101(1194):330-350. doi: 10.1093/postmj/qgae148. Postgrad Med J. 2025. PMID: 39487697
-
Cardiovascular outcomes trials: a paradigm shift in the current management of type 2 diabetes.Cardiovasc Diabetol. 2022 Aug 4;21(1):144. doi: 10.1186/s12933-022-01575-9. Cardiovasc Diabetol. 2022. PMID: 35927730 Free PMC article. Review.
Cited by
-
The right ventricular dysfunction and ventricular interdependence in patients with DM: assessment using cardiac MR feature tracking.Cardiovasc Diabetol. 2023 Apr 21;22(1):93. doi: 10.1186/s12933-023-01806-7. Cardiovasc Diabetol. 2023. PMID: 37085847 Free PMC article.
-
Efficacy of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists on proteinuria and weight in a diabetes cohort.World J Diabetes. 2025 Feb 15;16(2):98552. doi: 10.4239/wjd.v16.i2.98552. World J Diabetes. 2025. PMID: 39959283 Free PMC article.
-
Characterization of treatment intensified (add-on to metformin) adults with type 2 diabetes in Thailand: A cross-sectional real-world study (CONVERGE).J Diabetes Investig. 2025 Jun;16(6):1010-1019. doi: 10.1111/jdi.14409. Epub 2025 Mar 12. J Diabetes Investig. 2025. PMID: 40077899 Free PMC article.
-
Use of SGLT2 Inhibitors Versus DPP-4 Inhibitors and Age-Related Macular Degeneration in Patients WithType 2 Diabetes: A Multinational Cohort Study.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):58. doi: 10.1167/iovs.66.4.58. Invest Ophthalmol Vis Sci. 2025. PMID: 40257783 Free PMC article.
-
GLP-1RA and SGLT2i utilization in people with type 2 diabetes with atherosclerotic cardiovascular disease (ASCVD) or at high risk of ASCVD in the Gulf Region: Results from the PACT-MEA studys.Saudi Med J. 2025 Feb;46(2):163-170. doi: 10.15537/smj.2025.46.2.20240620. Saudi Med J. 2025. PMID: 39933762 Free PMC article.
References
-
- International Diabetes Federation Diabetes Atlas. 10th ed. Brussels: The Federation; 2021. https://www.diabetesatlas.org/data/en/world/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

