Inhibition of p38 MAPK or immunoproteasome overcomes resistance of chronic lymphocytic leukemia cells to Bcl-2 antagonist venetoclax
- PMID: 36209148
- PMCID: PMC9547871
- DOI: 10.1038/s41419-022-05287-6
Inhibition of p38 MAPK or immunoproteasome overcomes resistance of chronic lymphocytic leukemia cells to Bcl-2 antagonist venetoclax
Abstract
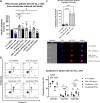
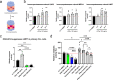
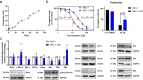
Chronic lymphocytic leukemia (CLL) is a hematological neoplasm of CD19-positive mature-appearing B lymphocytes. Despite the clinical success of targeted therapies in CLL, the development of resistance diminishes their therapeutic activity. This is also true for the Bcl-2 antagonist venetoclax. We investigated the molecular mechanisms that drive venetoclax resistance in CLL, with a clear focus to provide new strategies to successfully combat it. Activation of CLL cells with IFNγ, PMA/ionomycin, and sCD40L diminished the cytotoxicity of venetoclax. We demonstrated that the metabolic activity of cells treated with 1 nM venetoclax alone was 48% of untreated cells, and was higher for cells co-treated with IFNγ (110%), PMA/ionomycin (78%), and sCD40L (62%). As of molecular mechanism, we showed that PMA/ionomycin and sCD40L triggered translocation of NFκB in primary CLL cells, while IFNγ activated p38 MAPK, suppressed spontaneous and venetoclax-induced apoptosis and induced formation of the immunoproteasome. Inhibition of immunoproteasome with ONX-0914 suppressed activity of immunoproteasome and synergized with venetoclax against primary CLL cells. On the other hand, inhibition of p38 MAPK abolished cytoprotective effects of IFNγ. We demonstrated that venetoclax-resistant (MEC-1 VER) cells overexpressed p38 MAPK and p-Bcl-2 (Ser70), and underexpressed Mcl-1, Bax, and Bak. Inhibition of p38 MAPK or immunoproteasome triggered apoptosis in CLL cells and overcame the resistance to venetoclax of MEC-1 VER cells and venetoclax-insensitive primary CLL cells. In conclusion, the p38 MAPK pathway and immunoproteasome represent novel targets to combat venetoclax resistance in CLL.
© 2022. The Author(s).
Conflict of interest statement
Author H.P. has been involved as consultant for Abbvie. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

