Functional mining of novel terpene synthases from metagenomes
- PMID: 36209178
- PMCID: PMC9548185
- DOI: 10.1186/s13068-022-02189-9
Functional mining of novel terpene synthases from metagenomes
Abstract
Background: Terpenes are one of the most diverse and abundant classes of natural biomolecules, collectively enabling a variety of therapeutic, energy, and cosmetic applications. Recent genomics investigations have predicted a large untapped reservoir of bacterial terpene synthases residing in the genomes of uncultivated organisms living in the soil, indicating a vast array of putative terpenoids waiting to be discovered.
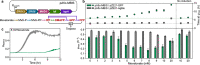
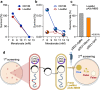
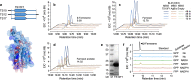
Results: We aimed to develop a high-throughput functional metagenomic screening system for identifying novel terpene synthases from bacterial metagenomes by relieving the toxicity of terpene biosynthesis precursors to the Escherichia coli host. The precursor toxicity was achieved using an inducible operon encoding the prenyl pyrophosphate synthetic pathway and supplementation of the mevalonate precursor. Host strain and screening procedures were finely optimized to minimize false positives arising from spontaneous mutations, which avoid the precursor toxicity. Our functional metagenomic screening of human fecal metagenomes yielded a novel β-farnesene synthase, which does not show amino acid sequence similarity to known β-farnesene synthases. Engineered S. cerevisiae expressing the screened β-farnesene synthase produced 120 mg/L β-farnesene from glucose (2.86 mg/g glucose) with a productivity of 0.721 g/L∙h.
Conclusions: A unique functional metagenomic screening procedure was established for screening terpene synthases from metagenomic libraries. This research proves the potential of functional metagenomics as a sequence-independent avenue for isolating targeted enzymes from uncultivated organisms in various environmental habitats.
Keywords: Functional metagenomics; Prenyl pyrophosphate; Terpene synthase; β-Farnesene.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage.Plant Physiol. 2002 Dec;130(4):2049-60. doi: 10.1104/pp.008326. Plant Physiol. 2002. PMID: 12481088 Free PMC article.
-
Enhancing structural diversity of terpenoids by multisubstrate terpene synthases.Beilstein J Org Chem. 2024 Apr 30;20:959-972. doi: 10.3762/bjoc.20.86. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38711588 Free PMC article. Review.
-
Production of multiple terpenes of different chain lengths by subcellular targeting of multi-substrate terpene synthase in plants.Metab Eng. 2020 Sep;61:397-405. doi: 10.1016/j.ymben.2020.08.002. Epub 2020 Aug 11. Metab Eng. 2020. PMID: 32795613
-
Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed.Biotechnol Bioeng. 2016 Jan;113(1):72-81. doi: 10.1002/bit.25683. Epub 2015 Sep 2. Biotechnol Bioeng. 2016. PMID: 26108688
-
Non-canonical substrates for terpene synthases in bacteria are synthesized by a new family of methyltransferases.FEMS Microbiol Rev. 2021 Nov 23;45(6):fuab024. doi: 10.1093/femsre/fuab024. FEMS Microbiol Rev. 2021. PMID: 33864462 Review.
Cited by
-
Decoding Catalysis by Terpene Synthases.ACS Catal. 2023 Sep 15;13(19):12774-12802. doi: 10.1021/acscatal.3c03047. eCollection 2023 Oct 6. ACS Catal. 2023. PMID: 37822860 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
