Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options
- PMID: 36209190
- PMCID: PMC9547753
- DOI: 10.1186/s40779-022-00422-y
Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options
Abstract
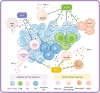
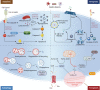
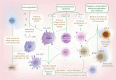
Sepsis is a common complication of combat injuries and trauma, and is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. It is also one of the significant causes of death and increased health care costs in modern intensive care units. The use of antibiotics, fluid resuscitation, and organ support therapy have limited prognostic impact in patients with sepsis. Although its pathophysiology remains elusive, immunosuppression is now recognized as one of the major causes of septic death. Sepsis-induced immunosuppression is resulted from disruption of immune homeostasis. It is characterized by the release of anti-inflammatory cytokines, abnormal death of immune effector cells, hyperproliferation of immune suppressor cells, and expression of immune checkpoints. By targeting immunosuppression, especially with immune checkpoint inhibitors, preclinical studies have demonstrated the reversal of immunocyte dysfunctions and established host resistance. Here, we comprehensively discuss recent findings on the mechanisms, regulation and biomarkers of sepsis-induced immunosuppression and highlight their implications for developing effective strategies to treat patients with septic shock.
Keywords: Immune monitoring; Immunomodulatory therapy; Immunosuppression; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

