Co-modulation of T cells and B cells enhances the inhibition of inflammation in experimental hypersensitivity pneumonitis
- PMID: 36209215
- PMCID: PMC9547367
- DOI: 10.1186/s12931-022-02200-9
Co-modulation of T cells and B cells enhances the inhibition of inflammation in experimental hypersensitivity pneumonitis
Abstract
Background: Hypersensitivity pneumonitis (HP) is an interstitial lung disease characterized by antigen-triggered neutrophilic exacerbations. Although CD4+ T cells are sufficient for HP pathogenesis, this never translated into efficient T cell-specific therapies. Increasing evidence shows that B cells also play decisive roles in HP. Here, we aimed to further define the respective contributions of B and T cells in subacute experimental HP.
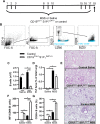
Methods: Mice were subjected to a protocol of subacute exposure to the archaeon Methanosphaera stadmanae to induce experimental HP. Using models of adoptive transfers of B cells and T cells in Rag1-deficient mice and of B cell-specific S1P1 deletion, we assessed the importance of B cells in the development of HP by evaluating inflammation in bronchoalveolar lavage fluid. We also aimed to determine if injected antibodies targeting B and/or T cells could alleviate HP exacerbations using a therapeutic course of intervention.
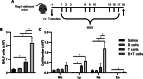
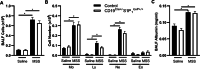
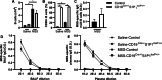
Results: Even though B cells are not sufficient to induce HP, they strongly potentiate CD4+ T cell-induced HP‑associated neutrophilic inflammation in the airways. However, the reduction of 85% of lung B cells in mice with a CD19-driven S1P1 deletion does not dampen HP inflammation, suggesting that lung B cells are not necessary in large numbers to sustain local inflammation. Finally, we found that injecting antibodies targeting B cells after experimental HP was induced does not dampen neutrophilic exacerbation. Yet, injection of antibodies directed against B cells and T cells yielded a potent 76% inhibition of neutrophilic accumulation in the lungs. This inhibition occurred despite partial, sometimes mild, depletion of B cells and T cells subsets.
Conclusions: Although B cells are required for maximal inflammation in subacute experimental HP, partial reduction of B cells fails to reduce HP-associated inflammation by itself. However, co-modulation of T cells and B cells yields enhanced inhibition of HP exacerbation caused by an antigenic rechallenge.
Keywords: Adoptive lymphocyte transfer; B cells; Biologics; CD69; Conditional knockout; Extrinsic allergic alveolitis; Hypersensitivity pneumonitis; Rituximab; S1P1.
© 2022. The Author(s).
Conflict of interest statement
DM received a funding from BMS for a COVID-19 project. There is no financial link with this project. The authors declare that they have no competing interests.
Figures



Similar articles
-
Impaired Immunosuppressive Effect of Bronchoalveolar Mesenchymal Stem Cells in Hypersensitivity Pneumonitis: Preliminary Findings.Cytometry B Clin Cytom. 2018 Mar;94(2):363-368. doi: 10.1002/cyto.b.21490. Epub 2016 Nov 17. Cytometry B Clin Cytom. 2018. PMID: 27792269
-
S1P1 Contributes to Endotoxin-enhanced B-Cell Functions Involved in Hypersensitivity Pneumonitis.Am J Respir Cell Mol Biol. 2020 Aug;63(2):209-218. doi: 10.1165/rcmb.2019-0339OC. Am J Respir Cell Mol Biol. 2020. PMID: 32289229 Free PMC article.
-
IL-4-secreting NKT cells prevent hypersensitivity pneumonitis by suppressing IFN-gamma-producing neutrophils.J Immunol. 2006 Oct 15;177(8):5258-68. doi: 10.4049/jimmunol.177.8.5258. J Immunol. 2006. PMID: 17015711
-
Bronchoalveolar Lavage Lymphocytes in the Diagnosis of Hypersensitivity Pneumonitis among Patients with Interstitial Lung Disease.Ann Am Thorac Soc. 2020 Nov;17(11):1455-1467. doi: 10.1513/AnnalsATS.202005-420OC. Ann Am Thorac Soc. 2020. PMID: 32757946
-
Hypersensitivity pneumonitis and alpha-chemokines.Clin Ter. 2017 Mar-Apr;168(2):e140-e145. doi: 10.7417/CT.2017.1996. Clin Ter. 2017. PMID: 28383627 Review.
Cited by
-
Candidatus Methanosphaera massiliense sp. nov., a methanogenic archaeal species found in a human fecal sample and prevalent in pigs and red kangaroos.Microbiol Spectr. 2024 Feb 6;12(2):e0514122. doi: 10.1128/spectrum.05141-22. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189277 Free PMC article.
-
Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand.Int J Mol Sci. 2025 Mar 29;26(7):3197. doi: 10.3390/ijms26073197. Int J Mol Sci. 2025. PMID: 40243992 Free PMC article.
-
The Effects of Interstitial Lung Diseases on Alveolar Extracellular Vesicles Profile: A Multicenter Study.Int J Mol Sci. 2023 Feb 17;24(4):4071. doi: 10.3390/ijms24044071. Int J Mol Sci. 2023. PMID: 36835481 Free PMC article.
References
-
- Schuyler M, Gott K, Shopp G, Crooks L. CD3+ AND CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Am Rev Respir Dis. 1992;146:1582–1588. - PubMed
-
- Schuyler M, Gott K, Edwards B, Nikula KJ. Experimental hypersensitivity pneumonitis - effect of CD4 cell depletion. Am J Respir Crit Care Med. 1994;149:1286–1294. - PubMed
-
- Denis M, Cormier Y, Laviolette M, Ghadirian E. T cells in hypersensitivity pneumonitis: effects of in vivo depletion of T cells in a mouse model. Am J Respir Cell Mol Biol. 1992;6:183–189. - PubMed
-
- Schuyler M, Gott K, Shopp G, Crooks L. CD3+, CD4+, CD8-, IA- T-cells adoptively transfer murine experimental hypersensitivity pneumonitis. Chest. 1993;103:S143–S145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

