Efficacy and Safety of Filgotinib in Patients with High Risk of Poor Prognosis Who Showed Inadequate Response to MTX: A Post Hoc Analysis of the FINCH 1 Study
- PMID: 36209441
- PMCID: PMC9931960
- DOI: 10.1007/s40744-022-00498-x
Efficacy and Safety of Filgotinib in Patients with High Risk of Poor Prognosis Who Showed Inadequate Response to MTX: A Post Hoc Analysis of the FINCH 1 Study
Erratum in
-
Correction: Efficacy and Safety of Filgotinib in Patients with High Risk of Poor Prognosis Who Showed Inadequate Response to MTX: A Post Hoc Analysis of the FINCH 1 Study.Rheumatol Ther. 2023 Feb;10(1):71-72. doi: 10.1007/s40744-022-00530-0. Rheumatol Ther. 2023. PMID: 36598735 Free PMC article. No abstract available.
Abstract
Introduction: This exploratory analysis of FINCH 1 (NCT02889796) examined filgotinib (FIL) efficacy and safety in a subgroup of patients with rheumatoid arthritis (RA) and inadequate response to methotrexate (MTX; MTX-IR) who had four poor prognostic factors (PPFs).
Methods: Patients with MTX-IR received placebo up to week (W)24 or FIL200 mg, FIL100 mg, or adalimumab up to W52; all received MTX. Efficacy and safety data were stratified by four PPFs versus fewer than four PPFs: seropositivity, high-sensitivity C-reactive protein (CRP) ≥ 6 mg/L, Disease Activity Score in 28 joints with CRP > 5.1, and erosions on X-rays.
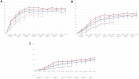
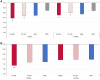
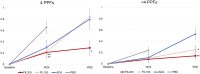
Results: At baseline, 687/1755 patients had four PPFs. At W12, whether with four PPFs or fewer than four PPFs, response rates on all American College of Rheumatology (ACR) measures were significantly greater with FIL200 and FIL100 versus placebo. At W52, FIL200 ACR20/50/70 response rates remained at least numerically higher versus adalimumab in both subgroups. At W52, FIL200 reduced modified total Sharp score (mTSS) change versus adalimumab in patients with four or fewer than four PPFs.
Conclusions: In high-risk (four PPFs) patients with MTX-IR RA, FIL200 and FIL100 showed similar reductions in disease activity versus placebo at W12 as in patients with fewer than four PPFs. mTSS in patients receiving FIL200 changed little from W24 to W52, while that in patients receiving FIL100 progressed comparably to patients who received adalimumab. Tolerability was comparable across treatment arms and subgroups.
Keywords: Adalimumab; Filgotinib; Methotrexate; Poor prognostic factors.
© 2022. The Author(s).
Figures


References
-
- Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

