Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer's Disease Neuroimaging Initiative 4
- PMID: 36209495
- PMCID: PMC10042173
- DOI: 10.1002/alz.12797
Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer's Disease Neuroimaging Initiative 4
Abstract
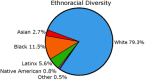
Introduction: The Alzheimer's Disease Neuroimaging Initiative (ADNI) aims to validate biomarkers for Alzheimer's disease (AD) clinical trials. To improve generalizability, ADNI4 aims to enroll 50-60% of its new participants from underrepresented populations (URPs) using new biofluid and digital technologies. ADNI4 has received funding from the National Institute on Aging beginning September 2022.
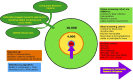
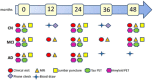
Methods: ADNI4 will recruit URPs using community-engaged approaches. An online portal will screen 20,000 participants, 4000 of whom (50-60% URPs) will be tested for plasma biomarkers and APOE. From this, 500 new participants will undergo in-clinic assessment joining 500 ADNI3 rollover participants. Remaining participants (∼3500) will undergo longitudinal plasma and digital cognitive testing. ADNI4 will add MRI sequences and new PET tracers. Project 1 will optimize biomarkers in AD clinical trials.
Results and discussion: ADNI4 will improve generalizability of results, use remote digital and blood screening, and continue providing longitudinal clinical, biomarker, and autopsy data to investigators.
Keywords: Alzheimer's disease; amyloid; cerebrovascular disease; digital biomarkers; generalizability; mild cognitive impairment; plasma biomarkers; tau; underrepresented populations.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Aisen reports research agreements with Janssen, Lilly, and Eisai; grants from NIA, the Alzheimer's Association, and FNIH; and consulting fees from Biogen, Roche, Merck, Abbvie, Immunobrain Checkpoint, Rainbow Medical, and Shionogi.
Dr. Albala has no conflicts to declare.
Dr. Beckett receives support from NIH grants U01AG024904, R01AG062517, and B639943, and has received support from the National Institute of Justice (2014‐R2‐CX‐0012).
Dr. Green is supported by NIH grants AG24904, HD090019, HG009922, HL143295, HG008685, TR003201, has received compensation for advising the following companies: AIA, Allelica, Embryome, Genomic Life, Grail, Humanity, Kneed Media, Meenta, OptumLabs, Plumcare, Verily, VinBigData; and is co‐founder of Genome Medical, Inc.
Dr. Harvey receives support from NIH grants P30AG072972, U01AG024904, U54NS079202, R01AG051618, R01HD093654, R01AG062240, R01AG062689, R01AG064688, P50HD103526, R01HD076189, R01AG066748, R01AG067541 and a California Department of Public Health Alzheimer's Award (1910611‐0). She has also served as a consultant for NervGen Pharma Corp and receives support for serving as a Statistical Advisor for PLOS ONE.
Dr. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH, the GHR Foundation, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic.
Dr. Jagust receives support from NIH grants AG034570, AG062542 and AG067418. He serves as a consultant to Bioclinica, Biogen, and Lilly and has an equity position in Optoceutics.
Dr. Landau has received travel funding from the Alzheimer's Association, served on the Scientific Advisory Board and DSMB for KeifeRx, and has received speaker fees from Eisai, Inc.
Dr. Miller has no conflicts to declare.
Dr. Morris is funded by NIH grants P30 AG066444, P01AG003991, P01AG026276, U19 AG032438, and U19 AG024904. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
Dr. Nosheny receives support in the form of grants to UCSF from NIH, The Alzheimer's Association, and Genentech, Inc.
Dr. Okonkwo has no conflicts to declare.
Dr. Perrin is supported by NIH grants R01AG068319, R01 AG053267, R01AG054567, P01 AG003991, P30 AG066444, U19AG024904, U19 AG032438, R01 AG052550, R01 AG070883, R01NS097799, R01NS092865, R01AG054513, and R01 NS075321. Neither Dr. Perrin nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
Dr. Petersen has consulted for Roche, Inc., Merck, Inc., Biogen, Inc., Eisai, Inc., Nestle, Inc., and Genentech, Inc.
Dr. Rivera Mindt receives support from multiple NIH grants (R01AG065110‐01A1, R01AG066471‐01A1, 5U19AG024904, R13 AG071313‐01, SC3GM141996) and the Genentech Health Equity Innovations 2020 Fund (G‐89294). She serves on the following Boards: ALL‐FTD External Advisory Board, Alzheimer's Association New York City, Brown University Carney Center, Harlem Community and Academic Partnership, South Texas Alzheimer's Disease Research Center (ADRC) External Advisory Board, and University of Washington ADRC External Advisory Board.
Dr. Saykin receives support from multiple NIH grants (P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U01 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, and U01 AG068057 and U01 AG072177). He has also received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor); Bayer Oncology (Scientific Advisory Board); Eisai (Scientific Advisory Board); Siemens Medical Solutions USA, Inc. (Dementia Advisory Board); and Springer‐Nature Publishing (Editorial Office Support as Editor‐in‐Chief, Brain Imaging and Behavior).
Dr. Shaw receives support from NIH grants P30 AG072979, U19AG024904, R01MH117114; grant support from the Michael J Fox Foundation for Parkinson's Disease Research and support from Roche (IIS and in‐kind reagents and instrumentation support for CSF AD biomarkers); he has received honoraria from Roche, Biogen, and Fujirebio for participation in teaching programs and served on Advisory Boards for Roche and Biogen.
Dr. Toga has no conflicts to declare.
Dr. Tosun receives support from NIH grant U01AG024904 and from the Department of Defense (grant numbers W81XWH‐12‐2‐0012, W81XWH‐13‐1‐0259, and W81XWH‐14‐1‐0462),
Dr. Veitch has no conflicts to declare.
Dr. Weiner serves on Editorial Boards for Alzheimer's & Dementia, MRI and TMRI. He has served on Advisory Boards for Acumen Pharmaceutical, ADNI, Alzheon, Inc., Biogen, Brain Health Registry, Cerecin, Dolby Family Ventures, Eli Lilly, Merck Sharp & Dohme Corp., National Institute on Aging (NIA), Nestle/Nestec, PCORI/PPRN, Roche, University of Southern California (USC), andNervGen. He has provided consulting to Baird Equity Capital, BioClinica, Cerecin, Inc., Cytox, Dolby Family Ventures, Duke University, Eisai, FUJIFILM‐Toyama Chemical (Japan), Garfield Weston, Genentech, Guidepoint Global, Indiana University, Japanese Organization for Medical Device Development, Inc. (JOMDD), Medscape, Nestle/Nestec, NIH, Peerview Internal Medicine, Roche, T3D Therapeutics, University of Southern California (USC), and Vida Ventures. He has acted as a speaker/lecturer to The Buck Institute for Research on Aging; China Association for Alzheimer's Disease (CAAD); Japan Society for Dementia Research; and Korean Dementia Society He holds stock options with Alzheon, Inc., Alzeca, and Anven. The following entities have provided funding for academic travel; University of Southern California (USC), NervGen, ASFNR, and CTAD Congress.
Author disclosures are available in the supporting information.
Figures
References
-
- Veitch DP, Weiner MW, Aisen PS, et al. Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2019;15:106‐152. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 HD093654/HD/NICHD NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- R01 AG051618/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- R01 AG062517/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R01 HD076189/HD/NICHD NIH HHS/United States
- U54 NS079202/NS/NINDS NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- R01 AG064688/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG062689/AG/NIA NIH HHS/United States
- P50 HD103526/HD/NICHD NIH HHS/United States
- R01 AG068193/AG/NIA NIH HHS/United States
- R01 AG062240/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous