The role of FXR and TGR5 in reversing and preventing progression of Western diet-induced hepatic steatosis, inflammation, and fibrosis in mice
- PMID: 36209823
- PMCID: PMC9638804
- DOI: 10.1016/j.jbc.2022.102530
The role of FXR and TGR5 in reversing and preventing progression of Western diet-induced hepatic steatosis, inflammation, and fibrosis in mice
Abstract
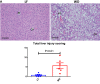
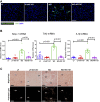
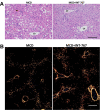
Nonalcoholic steatohepatitis (NASH) is the most common chronic liver disease in the US, partly due to the increasing incidence of metabolic syndrome, obesity, and type 2 diabetes. The roles of bile acids and their receptors, such as the nuclear receptor farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5, on the development of NASH are not fully clear. C57BL/6J male mice fed a Western diet (WD) develop characteristics of NASH, allowing determination of the effects of FXR and TGR5 agonists on this disease. Here we show that the FXR-TGR5 dual agonist INT-767 prevents progression of WD-induced hepatic steatosis, inflammation, and fibrosis, as determined by histological and biochemical assays and novel label-free microscopy imaging techniques, including third harmonic generation, second harmonic generation, and fluorescence lifetime imaging microscopy. Furthermore, we show INT-767 decreases liver fatty acid synthesis and fatty acid and cholesterol uptake, as well as liver inflammation. INT-767 markedly changed bile acid composition in the liver and intestine, leading to notable decreases in the hydrophobicity index of bile acids, known to limit cholesterol and lipid absorption. In addition, INT-767 upregulated expression of liver p-AMPK, SIRT1, PGC-1α, and SIRT3, which are master regulators of mitochondrial function. Finally, we found INT-767 treatment reduced WD-induced dysbiosis of gut microbiota. Interestingly, the effects of INT-767 in attenuating NASH were absent in FXR-null mice, but still present in TGR5-null mice. Our findings support treatment and prevention protocols with the dual FXR-TGR5 agonist INT-767 arrest progression of WD-induced NASH in mice mediated by FXR-dependent, TGR5-independent mechanisms.
Keywords: FXR-TGR5; NASH; bile acid; fibrosis; inflammation; lipid metabolism.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Dr Luciano Adorini is an employee of Intercept. These studies were in part supported by a medical school Investigator Initiated Study (IIS) grant to Moshe Levi. The authors otherwise have no other conflict of interest.
Figures














References
-
- Levene A.P., Goldin R.D. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152. - PubMed
-
- Wree A., Broderick L., Canbay A., Hoffman H.M., Feldstein A.E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013;10:627–636. - PubMed
-
- Marchesini G., Petta S., Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

