New strategies to improve clinical outcomes for diabetic kidney disease
- PMID: 36210442
- PMCID: PMC9548386
- DOI: 10.1186/s12916-022-02539-2
New strategies to improve clinical outcomes for diabetic kidney disease
Abstract
Background: Diabetic kidney disease (DKD), the most common cause of kidney failure and end-stage kidney disease worldwide, will develop in almost half of all people with type 2 diabetes. With the incidence of type 2 diabetes continuing to increase, early detection and management of DKD is of great clinical importance.
Main body: This review provides a comprehensive clinical update for DKD in people with type 2 diabetes, with a special focus on new treatment modalities. The traditional strategies for prevention and treatment of DKD, i.e., glycemic control and blood pressure management, have only modest effects on minimizing glomerular filtration rate decline or progression to end-stage kidney disease. While cardiovascular outcome trials of SGLT-2i show a positive effect of SGLT-2i on several kidney disease-related endpoints, the effect of GLP-1 RA on kidney-disease endpoints other than reduced albuminuria remain to be established. Non-steroidal mineralocorticoid receptor antagonists also evoke cardiovascular and kidney protective effects.
Conclusion: With these new agents and the promise of additional agents under clinical development, clinicians will be more able to personalize treatment of DKD in patients with type 2 diabetes.
Keywords: Diabetic kidney disease; Kidney protective agents; Type 2 diabetes.
© 2022. The Author(s).
Conflict of interest statement
TF provided advisory services to Astra Zeneca, Atrogi, Bayer, Cipla, Eli Lilly, Eysense, Fortbildungskolleg, Novo Nordisk, Pfizer, Sanofi, Remynd, and Roche. TF provided speaker services to Amarin, Astra Zeneca, Böhringer Ingelheim, Berlin Chemie, Cipla, Daiichi-Sankyo, Eli Lilly, Fortbildungskolleg, MSD, Novartis, Novo Nordisk, Sanofi, and Santis.
FG provided advisory services to AstraZeneca, Eli Lilly, Novo Nordisk, Roche Diabetes Care, and Sanofi; received speaker fees and served as a consultant for Boehringer Ingelheim, Lifescan, Merck Sharp & Dohme, Sanofi, AstraZeneca, Medimmune, Roche Diabetes Care, Sanofi, and Medtronic; and received research support from Eli Lilly and Roche Diabetes Care.
KRT is supported by NIH research grants R01MD014712, U2CDK114886, UL1TR002319, U54DK083912, U01DK100846, OT2HL161847, UM1AI109568, and CDC contract 75D301-21-P-12254; other support from Eli Lilly; personal fees and other support from Boehringer Ingelheim; personal fees and other support from AstraZeneca; grants, personal fees, and other support from Bayer AG; grants, personal fees, and other support from Novo Nordisk; grants and other support from Goldfinch Bio; other support from Gilead; and grants from Travere outside the submitted work.
RES is supported by grants from AstraZeneca, Boehringer Ingelheim, Lilly, and NovoNordisk to the Institution (University Hospital Eralngen); personal advisory and speaker fees were received from AstraZeneca, Bohringer Ingelheim, and NovoNordisk.
NP has been an advisory board member of AstraZeneca, Boehringer Ingelheim, MSD, NovoNordisk, Pfizer, Takeda, and TrigoCare International; has participated in sponsored studies by AstraZeneca, Eli Lilly, GSK, MSD, Novo Nordisk, Novartis, and Sanofi-Aventis; has received honoraria as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elpen, MSD, Mylan, NovoNordisk, Pfizer, Sanofi-Aventis, and Vianex; and attended conferences sponsored by TrigoCare International, Eli Lilly, Galenica, NovoNordisk, Pfizer, and Sanofi-Aventis.
DCW has an ongoing consultancy agreement with AstraZeneca. In the last 3 years, he has also received payments from Amgen, Astellas, Bayer, Boehringer Ingelheim, Janssen, Gilead, GlaxoSmithKline, Merck Sharp and Dohme, Mundipharma, Tricida, Vifor, and Zydus.
OS is founder and CEO of Sciarc GmbH, Germany.
CM serves or has served on the advisory panel for NovoNordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres, Mannkind, and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for CM from Medtronic, Imcyse, NovoNordisk, Sanofi, and ActoBio Therapeutics; CM serves or has served on the speaker’s bureau for NovoNordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven.
AH and MS declare no competing interests.
Figures
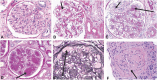


References
-
- International Diabetes Federation. IDF Diabetes Atlas. https://diabetesatlas.org/2021. Accessed 30 Aug 2022. - PubMed
-
- World Health Organization. Diabetes Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 30 Aug 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

