Synthesis of 7-Aminocoumarins from 7-Hydroxycoumarins via Amide Smiles Rearrangement
- PMID: 36211046
- PMCID: PMC9535735
- DOI: 10.1021/acsomega.2c04653
Synthesis of 7-Aminocoumarins from 7-Hydroxycoumarins via Amide Smiles Rearrangement
Abstract
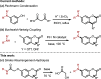
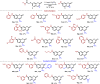
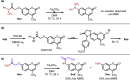
N-Substituted 7-aminocoumarins can be synthesized from readily available 7-hydroxycoumarins via alkylation with α-bromoacetamides and subsequent tandem O → N Smiles rearrangement-amide hydrolysis. The key rearrangement sequence proceeds under mild conditions to provide convenient access to various N-alkyl and N-aryl products in moderate to high yields. The process is operationally simple, inexpensive, transition-metal-free, and can be telescoped into a one-pot process.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
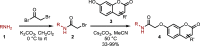
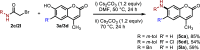

References
-
- Calcio Gaudino E. C.; Tagliapietra S.; Martina K.; Palmisano G.; Cravotto G. Recent Advances and Perspectives in the Synthesis of Bioactive Coumarins. RSC Adv. 2016, 6, 46394–46405. 10.1039/C6RA07071J. - DOI
LinkOut - more resources
Full Text Sources
