Mono- and Bimetallic Nanoparticles for Catalytic Degradation of Hazardous Organic Dyes and Antibacterial Applications
- PMID: 36211055
- PMCID: PMC9535655
- DOI: 10.1021/acsomega.2c03784
Mono- and Bimetallic Nanoparticles for Catalytic Degradation of Hazardous Organic Dyes and Antibacterial Applications
Abstract
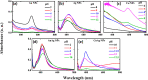
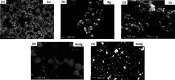
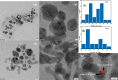
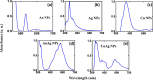
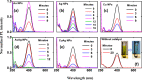
In the present work, gold (Au), silver (Ag), and copper (Cu) based mono- and bimetallic NPs are prepared using a cost-effective facile wet chemical route. The pH for the synthesis is optimized in accordance with the optical spectra and supported by the finite difference time domain simulation studies. FESEM and TEM micrographs are used to analyze the morphology of the prepared nanoparticles. TEM images of bimetallic nanoparticles (BMPs) verified their bimetallic nature. XRD studies confirmed the formation of fcc-structured mono- and bimetallic NPs. Photoluminescence studies of the as-synthesized NPs are in good agreement with the previous publications. These synthesized NPs showed enhanced catalytic activity for the reduction/degradation of 4-nitrophenol, rhodamine B, and indigo carmine dyes in the presence of sodium borohydride (NaBH4) compared to NaBH4 alone. For the reduction of 4-nitrophenol, Au, Cu, and CuAg nanoparticles exhibited good catalytic efficiency compared to others, whereas for the degradation of rhodamine B and indigo carmine dyes the catalytic efficiency is comparatively high for CuAg BMPs. Furthermore, the antibacterial assay is carried out, and Ag NPs display effective antibacterial activity against Klebsiella pneumoniae, Salmonella ser. Typhimurium, Acinetobacter baumannii, Shigella flexneri, and Pseudomonas aeruginosa.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Nowak A.; Szade J.; Talik E.; Zubko M.; Wasilkowski D.; Dulski M.; Balin K.; Mrozik A.; Peszke J. Physicochemical and Antibacterial Characterization of Ionocity Ag/Cu Powder Nanoparticles. Mater. Charact 2016, 117, 9–16. 10.1016/j.matchar.2016.04.013. - DOI
-
- He Z.; Zhang Z.; Bi S. Nanoparticles for Organic Electronics Applications. Mater. Res. Express 2020, 7 (1), 012004.10.1088/2053-1591/ab636f. - DOI
LinkOut - more resources
Full Text Sources
