Organocatalytic Reduction of Aromatic Nitro Compounds: The Use of Solid-Supported Phenyl(2-quinolyl)methanol
- PMID: 36211086
- PMCID: PMC9535724
- DOI: 10.1021/acsomega.2c04196
Organocatalytic Reduction of Aromatic Nitro Compounds: The Use of Solid-Supported Phenyl(2-quinolyl)methanol
Abstract
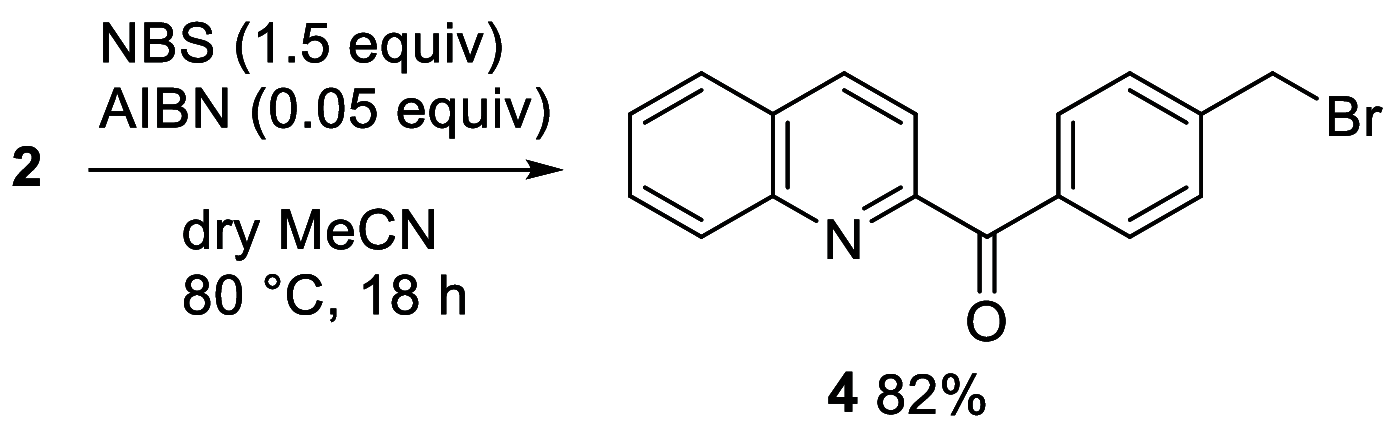
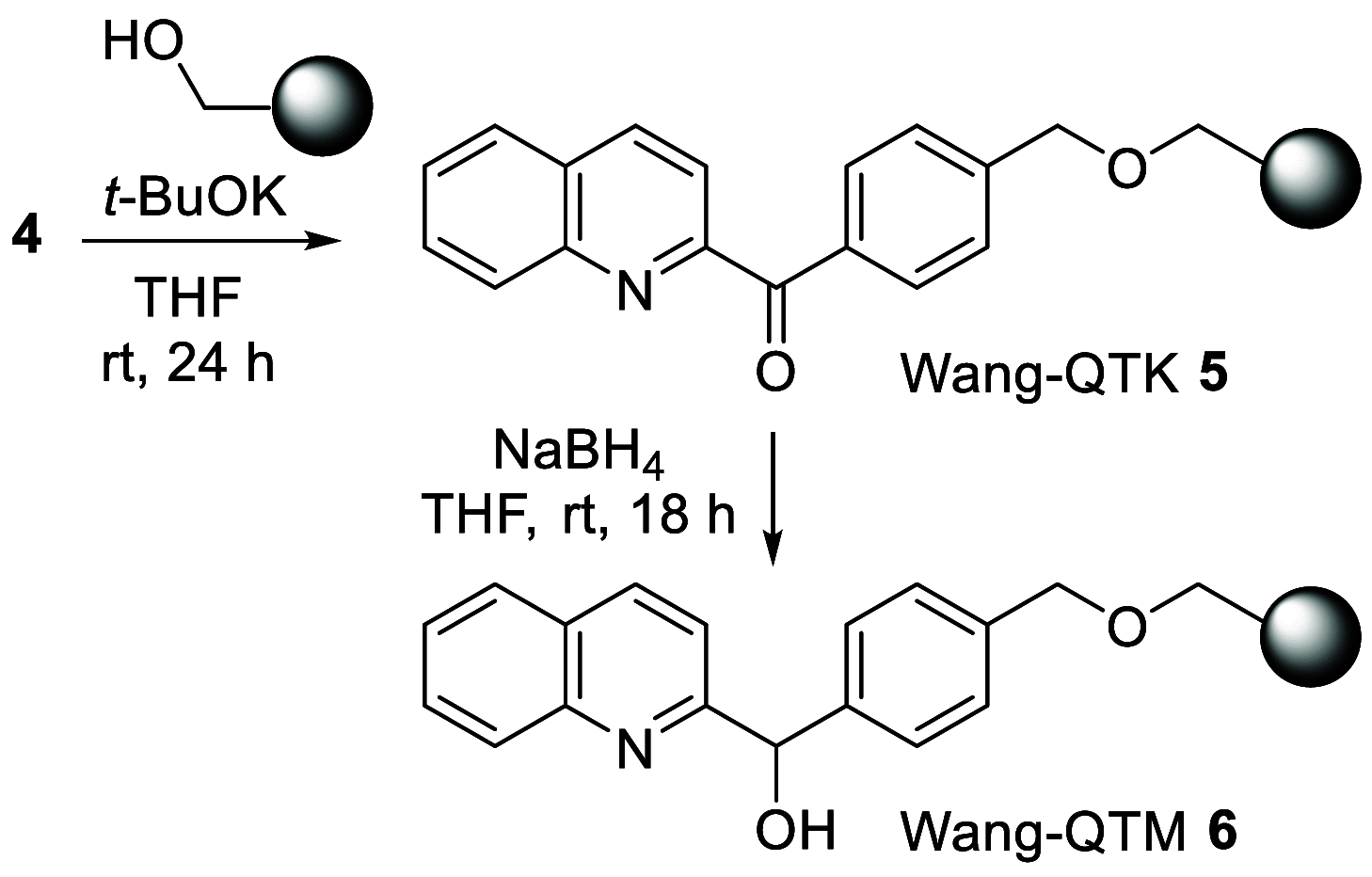
The reduction of aromatic nitro compounds has been performed employing a catalytic amount of Wang resin-supported phenyl(2-quinolyl)methanol (Wang-PQM) in the presence of an excess of NaBH4 to regenerate the reactive reducing species at the end of the process. The reduction products are easily isolated through a simple filtration/extraction protocol, and the catalyst can be efficiently recovered and recycled. The condensation route is generally preferred, and azo- and/or hydrazo-arenes can be easily prepared in high yields.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

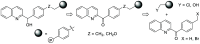


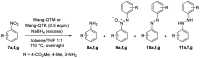
References
-
- Giomi D.; Alfini R.; Brandi A. New Reactivity of 1-(2-Pyridyl)-2-propen-1-ol with Nitro Derivatives. Tetrahedron Lett. 2008, 49, 6977–6979. 10.1016/j.tetlet.2008.09.105. - DOI
- Giomi D.; Piacenti M.; Alfini R.; Brandi A. 1-(2-Pyridyl)-2-propen-1-ol: a Multipurpose Reagent in Organic Synthesis. Tetrahedron 2009, 65, 7048–7055. 10.1016/j.tet.2009.06.044. - DOI
- Giomi D.; Alfini R.; Brandi A. (2-Pyridyl)phenylmethanol: a New Reagent for Metal-free Reduction of Nitro Aromatic Compounds. Tetrahedron 2011, 67, 167–172. 10.1016/j.tet.2010.11.008. - DOI
- Giomi D.; Alfini R.; Ceccarelli J.; Salvini A.; Brandi A. Phenyl(2-quinolyl)methanol: A Valuable Reagent for Metal-Free Reduction of Aromatic Nitro Compounds and Imines. ChemistrySelect 2016, 1, 5584–5589. 10.1002/slct.201601165. - DOI
-
- Giomi D.; Ceccarelli J.; Salvini A.; Brandi A. Organocatalytic Reduction of Nitroarenes with Phenyl(2-quinolyl)methanol. ChemistrySelect 2020, 5, 10511–10515. 10.1002/slct.202003234. - DOI
-
- Haber F. Über Stufenweise Reduktion des Nitrobenzol mit Begrenztem Kathodenpotential. Z. Elektrochem. 1898, 4, 506–514.
-
- Dai Y.; Li C.; Shen Y.; Lim T.; Xu J.; Li Y.; Niemantsverdriet H.; Besenbacher F.; Lock N.; Su R. Light-tuned Selective Photosynthesis of Azo- and Azoxy-aromatics Using Graphitic C3N4. Nat. Commun. 2018, 9, 60.and references therein10.1038/s41467-017-02527-8. - DOI - PMC - PubMed
- Breising V. M.; Kayser J. M.; Kehl A.; Schollmeyer D.; Liermann J. C.; Waldvogel S. R. Electrochemical Formation of N,N’-Diarylhydrazines by Dehydrogenative N–N Homocoupling Reaction. Chem. Commun. 2020, 56, 4348–4351. and references therein10.1039/D0CC01052A. - DOI - PubMed
-
- Aronzon D.; Levy E. P.; Collings P. J.; Chanishvili A.; Chilaya G.; Petriashvili G. Trans–cis Isomerization of an Azoxybenzene Liquid Crystal. Liq. Cryst. 2007, 34, 707–718. 10.1080/02678290701267480. - DOI
- Folcia C. L.; Alonso I.; Ortega J.; Etxebarria J.; Pintre I.; Ros M. B. Achiral Bent-Core Liquid Crystals with Azo and Azoxy Linkages: Structural and Nonlinear Optical Properties and Photoisomerization. Chem. Mater. 2006, 18, 4617–4626. 10.1021/cm060256p. - DOI
- Yu B.-C.; Shirai Y.; Tour J. M. Syntheses of New Functionalized Azobenzenes for Potential Molecular Electronic Devices. Tetrahedron 2006, 62, 10303–10310. 10.1016/j.tet.2006.08.069. - DOI
- Vix A. B. E.; Müller-Buschbaum P.; Stocker W.; Stamm M.; Rabe J. P. Crossover between Dewetting and Stabilization of Ultrathin Liquid Crystalline Polymer Films. Langmuir 2000, 16, 10456–10462. 10.1021/la000824u. - DOI
- Huang J. M.; Kuo J. F.; Chen C. Y. Studies on Mesomorphic Behaviors of Segmented Azoxy Polyester Containing Polyoxyethylene. J. Appl. Polym. Sci. 1995, 55, 1217–1229. 10.1002/app.1995.070550809. - DOI
LinkOut - more resources
Full Text Sources
