Optimal administration time of vitamin C after 131I therapy in differentiated thyroid cancer based on propensity score matching
- PMID: 36211303
- PMCID: PMC9535083
- DOI: 10.3389/fsurg.2022.993712
Optimal administration time of vitamin C after 131I therapy in differentiated thyroid cancer based on propensity score matching
Abstract
Objectives: This study aimed to investigate the protection of the salivary glands by vitamin C administration at 2 and 24 h after an initial treatment using iodine-131 (131I) in patients with differentiated thyroid cancer (DTC) and examined the optimal administration time of vitamin C to protect the salivary glands from radiation injury.
Method: The clinical data of patients with differentiated thyroid carcinoma who had been treated with 131I in the Department of Nuclear Medicine in Shanxi Bethune Hospital from January 2014 to December 2020 were retrospectively analyzed. The propensity score matching method was adopted to match patients who received the administration of vitamin C at 2 h with those receiving administration at 24 h. A total of 230 pairs/460 patients were enrolled in the study. The chi-squared (χ 2) or Fisher's exact test was used to compare the indicators representing the incidence of salivary gland injury between the two groups.
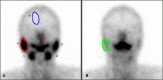
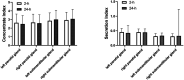
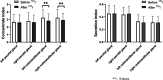
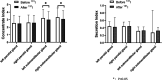
Results: The incidence of salivary gland injury (17.39%) with acidic substances at 2 h was lower compared with administration at 24 h (26.96%). The incidence of acute salivary gland injury (15.22%) and chronic salivary gland injury (26.09%) in the 24-h group were higher than those in the 2-h group (4.78% and 18.26%, respectively). The differences in the left submandibular gland concentrate index and right submandibular gland concentrate index were statistically significant before and after treatment in both the 2 and the 24-h groups; these functions had been impaired after treatment.
Conclusions: Following treatment with 131I, the protective effect of acidic substances administered at 2 and 24 h on the salivary glands were different. The incidence of salivary gland injury in the 2 h acid stimulation group was lower than in the 24 h acid stimulation group. The present study revealed that 131I treatment did cause some injury to the salivary glands and that the protective effect of administering vitamin C at 2 and 24 h may be limited. Accordingly, protection against salivary gland injury should be conducted using comprehensive measures.
Keywords: radioiodine therapy; salivary glands; sialadenitis; thyroid cancer; vitamin c.
© 2022 Liu, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Radioprotective effect of vitamin E on salivary glands after radioiodine therapy for differentiated thyroid cancer: a randomized-controlled trial.Nucl Med Commun. 2017 Nov;38(11):891-903. doi: 10.1097/MNM.0000000000000727. Nucl Med Commun. 2017. PMID: 28806348 Clinical Trial.
-
Effect of selenium supplementation for protection of salivary glands from iodine-131 radiation damage in patients with differentiated thyroid cancer.Hell J Nucl Med. 2017 Jan-Apr;20(1):62-70. doi: 10.1967/s002449910508. Epub 2017 Mar 20. Hell J Nucl Med. 2017. PMID: 28315910 Clinical Trial.
-
Effect of vitamin E and supragingival scaling on salivary gland function in patients with differentiated thyroid cancer treated with 131I.Nucl Med Commun. 2022 Sep 1;43(9):995-1003. doi: 10.1097/MNM.0000000000001605. Epub 2022 Aug 10. Nucl Med Commun. 2022. PMID: 35950355 Clinical Trial.
-
Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials.Front Endocrinol (Lausanne). 2022 Aug 29;13:960265. doi: 10.3389/fendo.2022.960265. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36105397 Free PMC article.
-
Sialadenitis as a complication of radioiodine therapy in patients with thyroid cancer: where do we stand?Hormones (Athens). 2021 Dec;20(4):669-678. doi: 10.1007/s42000-021-00304-3. Epub 2021 Jun 18. Hormones (Athens). 2021. PMID: 34143403 Review.
Cited by
-
Vitamin C in the Management of Thyroid Cancer: A Highway to New Treatment?Antioxidants (Basel). 2024 Oct 15;13(10):1242. doi: 10.3390/antiox13101242. Antioxidants (Basel). 2024. PMID: 39456495 Free PMC article. Review.
-
Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis.Cancers (Basel). 2023 Apr 29;15(9):2558. doi: 10.3390/cancers15092558. Cancers (Basel). 2023. PMID: 37174023 Free PMC article.
-
The Impact of Radioiodine (131I) Therapy of Thyroid Disease on Salivary Glands Function and Inflammation: A Comprehensive Review.Biomedicines. 2025 Jun 7;13(6):1404. doi: 10.3390/biomedicines13061404. Biomedicines. 2025. PMID: 40564122 Free PMC article. Review.
References
-
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. 10.1089/thy.2015.0020 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

