Biological drugs for systemic lupus erythematosus or active lupus nephritis and rates of infectious complications. Evidence from large clinical trials
- PMID: 36211360
- PMCID: PMC9538665
- DOI: 10.3389/fimmu.2022.999704
Biological drugs for systemic lupus erythematosus or active lupus nephritis and rates of infectious complications. Evidence from large clinical trials
Abstract
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease that frequently affects the kidneys, known as lupus nephritis (LN). Such patients are treated with antimalarials, corticosteroids or immunosuppressive drugs, and more recently, target-specific biological drugs. Although efficacy of these therapies improved SLE-related outcomes, SLE remains associated with higher rates of infections. Here, we performed a comprehensive systemic review of infectious complications in clinical trials covering drug interventions for SLE or specifically for active LN. Our search in 15 online registries yielded a total of 1477 studies of which 14 matched our prespecified criteria. These covered the biological drugs anifrolumab, belimumab, and rituximab that were tested in patients with non-renal SLE and active LN.The available safety data from the SLE trials indicated that infectious complications such as herpes zoster, upper respiratory tract infection, nasopharyngitis, bronchitis, and urinary tract infection in patients receiving placebo were quite prevalent especially in the EXPLORER (rituximab) trial. Infections occurred mostly during the first year of LN therapy. Serious adverse events and infectious complications occurred more frequently in placebo-treated patients with active LN, especially in the BLISS-LN (belimumab) and LUNAR (rituximab) trials. Anifrolumab and rituximab increased the number of clinically relevant episodes of herpes zoster compared to belimumab in patients with active LN. Anifrolumab displayed a similar trend for influenza infections, which is consistent with the specific mechanisms-of-action of anifrolumab; highlighting drug-specific effects on infectious complications. In addition, standard-of-care therapy, e.g., MMF and immunosuppressants, as well as a longer SLE duration may also affect the incidence of serious adverse events and certain infectious complications in SLE patients with active LN.Infectious complications are common in SLE but even more common in patients with active LN, especially herpes zoster is strongly associated with active LN and anifrolumab therapy (OR 2.8, 95% CI 1.18 to 6.66, p = 0.018). Immunotherapy seems to impose unspecific and specific risks for infections. The latter may imply specific precautions such as preemptive vaccination and individual risk-benefit assessments.
Keywords: anifrolumab; belimumab; chronic kidney disease (CKD); herpes zoster; infection; lupus; rituximab.
Copyright © 2022 Steiger, Ehreiser, Anders and Anders.
Conflict of interest statement
H-JA received consultancy or lecture fees from Boehringer, Bayer, GSK, AstraZeneca, Novartis, Otsuka, Janssen, Kezar, Lilly, Sanofi, and PreviPharma. SS has received research funding from Eleva Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
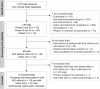
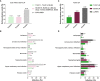

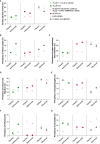
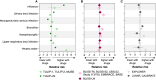
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

