Target organ expression and biomarker characterization of chemokine CCL21 in systemic sclerosis associated pulmonary arterial hypertension
- PMID: 36211384
- PMCID: PMC9541617
- DOI: 10.3389/fimmu.2022.991743
Target organ expression and biomarker characterization of chemokine CCL21 in systemic sclerosis associated pulmonary arterial hypertension
Abstract
Introduction: Systemic sclerosis (SSc) is a heterogenous disorder that appears to result from interplay between vascular pathologies, tissue fibrosis and immune processes, with evidence for deregulation of chemokines, which normally control immune trafficking. We recently identified altered levels of chemokine CCL21 in SSc associated pulmonary arterial hypertension (PAH). Here, we aimed to define target organ expression and biomarker characteristics of CCL21.
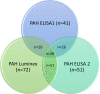
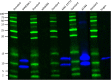
Materials and methods: To investigate target organ expression of CCL21, we performed immunohistochemistry (IHC) on explanted lung tissues from SSc-PAH patients. We assessed serum levels of CCL21 by ELISA and Luminex in two well-characterized SSc cohorts from Oslo (OUH, n=552) and Zurich (n=93) University hospitals and in 168 healthy controls. For detection of anti-CCl21 antibodies, we performed protein array analysis applying serum samples from SSc patients (n=300) and healthy controls. To characterize circulating CCL21 in SSc, we applied immunoprecipitation (IP) with antibodies detecting both full length and tailless and a custom-made antibody detecting only the C-terminal of CCL21. IP products were analyzed by SDS-PAGE/western blot and Mass spectrometry (MS).
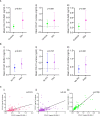
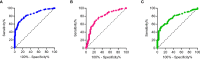
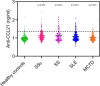
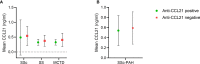
Results: By IHC, we found that CCL21 was mainly expressed in the airway epithelial cells of SSc patients with PAH. In the analysis of serum levels of CCL21 we found weak correlation between Luminex and ELISA (r=0.515, p<0.001). Serum levels of anti-CCL21 antibodies were higher in SSc patients than in healthy controls (p<0.001), but only 5% of the SSc population were positive for anti-CCL21 antibodies in SSc, and we found no correlation between anti-CCl21 and serum levels of CCL21. By MS, we only identified peptides located within amino acid (aa) 23-102 of CCL21, indicating that CCL21 in SSc circulate as a truncated protein without the C-terminal tail.
Conclusion: This study demonstrates expression of CCL21 in epithelial lung tissue from SSc patients with PAH, and indicate that CCL21 in SSc circulates as a truncated protein. We extend previous observations indicating biomarker potential of CCL21, but find that Luminex is not suitable as platform for biomarker analyses. Finally, in vivo generated anti-CCL21 antibodies exist in SSc, but do not appear to modify serum CCL21 levels in patients with SSc-PAH.
Keywords: Anti-CCL21; CCL21; ELISA Enzyme-linked immunosorbent assay; Luminex (xMAP) method; pulmonary arteria hypertension; systemic sclerosis.
Copyright © 2022 Didriksen, Molberg, Mehta, Jordan, Palchevskiy, Fretheim, Gude, Ueland, Brunborg, Garen, Midtvedt, Andreassen, Lund-Johansen, Distler, Belperio and Hoffmann-Vold.
Conflict of interest statement
HF has received personal fees from Bayer and non-financial support <$10 000 from GSK and Actelion, outside the submitted work. ØMi has received honoraria from Boehringer Ingelheim and Jannsen. AA has a consultancy relationship with and has received travel support and honoraria from Jannsen. OD has a consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, AstraZeneca, Baecon, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, 4P Science, Galapagos, Glenmark, Horizon, Inventiva, Janssen, Kymera, Lupin, Medscape, Miltenyi Biotec, Mitsubishi Tanabe, MSD, Novartis, Prometheus, Redxpharma, Roivant, Sanofi and Topadur. OD also has a patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). JB has received honoraria from Boehringer Ingelheim. A-MH-V has received grants, personal fees and non-financial support >$10 000 from Boehringer Ingelheim, and personal fees <$10 000 from Actelion, ARXX, Bayer, Jansen, Medscape, MSD and Roche outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
CCL21 as a Potential Serum Biomarker for Pulmonary Arterial Hypertension in Systemic Sclerosis.Arthritis Rheumatol. 2018 Oct;70(10):1644-1653. doi: 10.1002/art.40534. Epub 2018 Aug 30. Arthritis Rheumatol. 2018. PMID: 29687634
-
Interleukin-32 in systemic sclerosis, a potential new biomarker for pulmonary arterial hypertension.Arthritis Res Ther. 2020 Jun 1;22(1):127. doi: 10.1186/s13075-020-02218-8. Arthritis Res Ther. 2020. PMID: 32487240 Free PMC article.
-
Soluble markers of B cell activation suggest a role of B cells in the pathogenesis of systemic sclerosis-associated pulmonary arterial hypertension.Front Immunol. 2022 Jul 29;13:954007. doi: 10.3389/fimmu.2022.954007. eCollection 2022. Front Immunol. 2022. PMID: 35967377 Free PMC article.
-
Screening for the early detection of pulmonary arterial hypertension in patients with systemic sclerosis: A systematic review and meta-analysis of long-term outcomes.Semin Arthritis Rheum. 2021 Jun;51(3):495-512. doi: 10.1016/j.semarthrit.2021.03.011. Epub 2021 Apr 4. Semin Arthritis Rheum. 2021. PMID: 33857705
-
Vascular endothelial injury assessed with functional techniques in systemic sclerosis patients with pulmonary arterial hypertension versus systemic sclerosis patients without pulmonary arterial hypertension: a systematic review and meta-analysis.Rheumatol Int. 2021 Jun;41(6):1045-1053. doi: 10.1007/s00296-021-04850-2. Epub 2021 Apr 8. Rheumatol Int. 2021. PMID: 33830321
Cited by
-
Current Trends in Vascular Biomarkers for Systemic Sclerosis: A Narrative Review.Int J Mol Sci. 2023 Feb 17;24(4):4097. doi: 10.3390/ijms24044097. Int J Mol Sci. 2023. PMID: 36835506 Free PMC article. Review.
-
Identification of potential biomarkers for idiopathic pulmonary arterial hypertension using single-cell and bulk RNA sequencing analysis.Front Genet. 2024 Mar 22;15:1328234. doi: 10.3389/fgene.2024.1328234. eCollection 2024. Front Genet. 2024. PMID: 38586587 Free PMC article.
-
Serum Biomarkers in Connective Tissue Disease-Associated Pulmonary Arterial Hypertension.Int J Mol Sci. 2023 Feb 20;24(4):4178. doi: 10.3390/ijms24044178. Int J Mol Sci. 2023. PMID: 36835590 Free PMC article. Review.
-
Biomarkers in the Pathogenesis, Diagnosis, and Treatment of Systemic Sclerosis.J Inflamm Res. 2023 Oct 17;16:4633-4660. doi: 10.2147/JIR.S379815. eCollection 2023. J Inflamm Res. 2023. PMID: 37868834 Free PMC article. Review.
-
A comprehensive analysis of genes associated with hypoxia and cuproptosis in pulmonary arterial hypertension using machine learning methods and immune infiltration analysis: AHR is a key gene in the cuproptosis process.Front Med (Lausanne). 2024 Sep 26;11:1435068. doi: 10.3389/fmed.2024.1435068. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39391037 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

