Discovery and characterization of SARS-CoV-2 reactive and neutralizing antibodies from humanized CAMouseHG mice through rapid hybridoma screening and high-throughput single-cell V(D)J sequencing
- PMID: 36211410
- PMCID: PMC9545174
- DOI: 10.3389/fimmu.2022.992787
Discovery and characterization of SARS-CoV-2 reactive and neutralizing antibodies from humanized CAMouseHG mice through rapid hybridoma screening and high-throughput single-cell V(D)J sequencing
Abstract
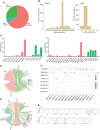
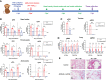
The coronavirus disease 2019 pandemic has caused more than 532 million infections and 6.3 million deaths to date. The reactive and neutralizing fully human antibodies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are effective detection tools and therapeutic measures. During SARS-CoV-2 infection, a large number of SARS-CoV-2 reactive and neutralizing antibodies will be produced. Most SARS-CoV-2 reactive and neutralizing fully human antibodies are isolated from human and frequently encoded by convergent heavy-chain variable genes. However, SARS-CoV-2 viruses can mutate rapidly during replication and the resistant variants of neutralizing antibodies easily survive and evade the immune response, especially in the face of such focused antibody responses in humans. Therefore, additional tools are needed to develop different kinds of fully human antibodies to compensate for current deficiency. In this study, we utilized antibody humanized CAMouseHG mice to develop a rapid antibody discovery method and examine the antibody repertoire of SARS-CoV-2 RBD-reactive hybridoma cells derived from CAMouseHG mice by using high-throughput single-cell V(D)J sequencing analysis. CAMouseHG mice were immunized by 28-day rapid immunization method. After electrofusion and semi-solid medium screening on day 12 post-electrofusion, 171 hybridoma clones were generated based on the results of SARS-CoV-2 RBD binding activity assay. A rather obvious preferential usage of IGHV6-1 family was found in these hybridoma clones derived from CAMouseHG mice, which was significantly different from the antibodies found in patients with COVID-19. After further virus neutralization screening and antibody competition assays, we generated a noncompeting two-antibody cocktail, which showed a potent prophylactic protective efficacy against SARS-CoV-2 in cynomolgus macaques. These results indicate that humanized CAMouseHG mice not only provide a valuable platform to obtain fully human reactive and neutralizing antibodies but also have a different antibody repertoire from humans. Thus, humanized CAMouseHG mice can be used as a good complementary tool in discovery of fully human therapeutic and diagnostic antibodies.
Keywords: SARS-CoV-2; antibody humanized mice; fully human antibody; reactive and neutralizing antibodies; single-cell sequencing.
Copyright © 2022 Yang, Chi, Wu, Wang, Lang, Han, Wang, Liu, Li, Wang, Huang, Bi, Liang, Gao, Zhao, Feng, Yang, Wang, Xia and Ge.
Conflict of interest statement
Author MW is part-time employed by Chongqing Jinmaibo Bio. MW, LG and XL are shareholders of Chongqing Jinmaibo Bio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
A high-throughput single cell-based antibody discovery approach against the full-length SARS-CoV-2 spike protein suggests a lack of neutralizing antibodies targeting the highly conserved S2 domain.Brief Bioinform. 2022 May 13;23(3):bbac070. doi: 10.1093/bib/bbac070. Brief Bioinform. 2022. PMID: 35362510
-
Generation and characterization of humanized synergistic neutralizing antibodies against SARS-CoV-2.J Med Virol. 2022 Aug;94(8):3791-3800. doi: 10.1002/jmv.27801. Epub 2022 May 7. J Med Virol. 2022. PMID: 35451094 Free PMC article.
-
The spike-ACE2 binding assay: An in vitro platform for evaluating vaccination efficacy and for screening SARS-CoV-2 inhibitors and neutralizing antibodies.J Immunol Methods. 2022 Apr;503:113244. doi: 10.1016/j.jim.2022.113244. Epub 2022 Feb 23. J Immunol Methods. 2022. PMID: 35218866 Free PMC article.
-
Broad strategies for neutralizing SARS-CoV-2 and other human coronaviruses with monoclonal antibodies.Sci China Life Sci. 2023 Apr;66(4):658-678. doi: 10.1007/s11427-022-2215-6. Epub 2022 Nov 24. Sci China Life Sci. 2023. PMID: 36443513 Free PMC article. Review.
-
SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment.Annu Rev Med. 2022 Jan 27;73:1-16. doi: 10.1146/annurev-med-042420-113838. Epub 2021 Aug 24. Annu Rev Med. 2022. PMID: 34428080 Review.
Cited by
-
A rabies virus-vectored vaccine expressing two copies of the Marburg virus glycoprotein gene induced neutralizing antibodies against Marburg virus in humanized mice.Emerg Microbes Infect. 2023 Dec;12(1):2149351. doi: 10.1080/22221751.2022.2149351. Emerg Microbes Infect. 2023. PMID: 36453198 Free PMC article.
-
Differential detection workflows for multi-sample single-cell RNA-seq data.bioRxiv [Preprint]. 2023 Dec 19:2023.12.17.572043. doi: 10.1101/2023.12.17.572043. bioRxiv. 2023. PMID: 38187695 Free PMC article. Preprint.
-
Development and validation of a blocking ELISA for measurement of rabies virus neutralizing antibody.J Clin Microbiol. 2025 Jun 11;63(6):e0204924. doi: 10.1128/jcm.02049-24. Epub 2025 May 14. J Clin Microbiol. 2025. PMID: 40366189 Free PMC article.
-
High-throughput strategies for monoclonal antibody screening: advances and challenges.J Biol Eng. 2025 May 8;19(1):41. doi: 10.1186/s13036-025-00513-z. J Biol Eng. 2025. PMID: 40340930 Free PMC article. Review.
-
Fully human monoclonal antibodies against Ebola virus possess complete protection in a hamster model.Emerg Microbes Infect. 2024 Dec;13(1):2392651. doi: 10.1080/22221751.2024.2392651. Epub 2024 Aug 26. Emerg Microbes Infect. 2024. PMID: 39155772 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

