Transient Vision Loss Associated with Prefilled Aflibercept Syringes: A Case Series and Analysis of Injection Force
- PMID: 36211641
- PMCID: PMC9541561
- DOI: 10.1016/j.xops.2022.100115
Transient Vision Loss Associated with Prefilled Aflibercept Syringes: A Case Series and Analysis of Injection Force
Abstract
Objective: To describe cases of significant vision loss following intravitreal aflibercept using pre-filled syringes (PFS) and to study the relationship between syringe design, injection speed, and injection force.
Design: Retrospective case series and experimental study.
Subjects: 12 patients who received intravitreal aflibercept PFS.
Methods: All retina specialists (N=13) at Oregon Health & Science University and the Veterans Affairs Portland Medical Center were queried in December 2020 to report episodes of significant vision loss following aflibercept PFS use. Chart review was completed for all affected patients for demographics and pertinent ocular history. Using a commercially available force measuring system, injection force was measured for aflibercept PFS, ranibizumab PFS, and a tuberculin syringe at various injection speeds.
Main outcome measures: Number of significant vision loss episodes following aflibercept PFS use; average injection force (Newton, N) at various injection speeds across different syringes.
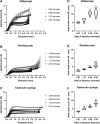
Results: Ten specialists (76.9%) reported a perceived increase in post-injection vision loss with aflibercept PFS. Three specialists had no cases of vision loss. 16 events of light perception or worse vision immediately following aflibercept PFS use were reported. Chart review was available for 12 of these events. The indication for aflibercept was exudative age-related macular degeneration (N=8), diabetic macular edema (N=3), and central serous chorioretinopathy (N=1). The median age of affected patients was 71 years (range 49-94). Two patients were being treated for glaucoma (N=1) or ocular hypertension (N=1); one patient was a glaucoma suspect. Anterior chamber paracentesis was performed in four patients to normalize intraocular pressure (IOP) promptly. Laboratory experiments demonstrated that higher injection speeds were associated with higher injection forces for all syringe types. Injection forces were consistently greater with aflibercept PFS than with the ranibizumab PFS or tuberculin syringes (p < 0.0001).
Conclusions: Retina specialists within our institutions have noted numerous cases of severe transient vision loss with aflibercept PFS use. Some affected patients have reported increased injection-related anxiety. The average injection force may be greater with the aflibercept PFS when compared to other intravitreal anti-VEGF options. Additional clinical studies are needed to better understand how syringe design and fluid dynamics may contribute to post-injection vision loss.
Conflict of interest statement
Conflicts of Interest: No conflicting relationship exists for any author.
Figures



References
-
- Eylea (aflibercept). U.S. Food and Drug Administration, 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125387s061lbl.pdf. Accessed August 1, 2021.
-
- Regeneron. FDA approves EYLEA (aflibercept) injection prefilled syringe. Tarrytown, New York: PRNewswire, 2019; v. 2020. Available at: https://www.prnewswire.com/news-releases/fda-approves-eylea-aflibercept-.... Accessed August 1, 2021.
Grants and funding
LinkOut - more resources
Full Text Sources

