Phenolic compositions and antioxidant activities of Hippophae tibetana and H. rhamnoides ssp. sinensis berries produced in Qinghai-Tibet Plateau
- PMID: 36211784
- PMCID: PMC9532713
- DOI: 10.1016/j.fochx.2022.100397
Phenolic compositions and antioxidant activities of Hippophae tibetana and H. rhamnoides ssp. sinensis berries produced in Qinghai-Tibet Plateau
Abstract
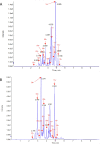
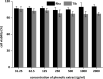
Phenolic ingredients of Hippophae tibetana (Tib) and H. rhamnoides ssp. sinensis (Rha) berry from Qinghai-Tibet Plateau were identified by Ultra Performance Liquid Chromatography-triple Quadrupole Tandem Mass Spectrometry. Results demonstrated that both of them possessed high levels of total phenolic and flavonoid, and compared to Tib, Rha berry exhibited higher contents. Moreover, flavonols was the most predominant subclass in Rha berry, flavonols and flavanols were the two most abundant subclasses in Tib berry. Among them, rutin and narcissin were present in the most abundant amounts in Rha berry, while (-)-epigallocatechin was the richest substance in Tib berry. Furthermore, both phenolic extracts of sea buckthorn berry exhibited strong in vitro and cellular antioxidant properties. Rha berry extract exhibited much stronger effects because of its higher levels of phenolic and flavonoid profiles. This finding proved that the Rha berry could serve as a food source for better health with great potential antioxidant activity.
Keywords: Antioxidant activity; Flavonoids; H. rhamnoides ssp. sinensis; Hippophae tibetana; Phenolic compounds; Sea buckthorn.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Antolovich M., Prenzler P.D., Patsalides E., McDonald S., Robards K. Methods for testing antioxidant activity. Analyst. 2002;127(1):183–198. - PubMed
-
- Arimboor R., Arumughan C. Effect of polymerization on antioxidant and xanthine oxidase inhibitory potential of sea buckthorn (H. rhamnoides) proanthocyanidins. Journal of Food Science. 2012;77(10):C1036–C1041. - PubMed
-
- Boudet A.M. Evolution and current status of research in phenolic compounds. Phytochemistry. 2007;68(22–24):2722–2735. - PubMed
-
- Fatima T., Kesari V., Watt I., Wishart D., Todd J.F., Schroeder W.R.…Krishna P. Metabolite profiling and expression analysis of flavonoid, vitamin C and tocopherol biosynthesis genes in the antioxidant-rich sea buckthorn (Hippophae rhamnoides L.) Phytochemistry. 2015;118:181–191. - PubMed
LinkOut - more resources
Full Text Sources

