Meningococcal virulence in zebrafish embryos depends on capsule polysaccharide structure
- PMID: 36211969
- PMCID: PMC9538531
- DOI: 10.3389/fcimb.2022.1020201
Meningococcal virulence in zebrafish embryos depends on capsule polysaccharide structure
Abstract
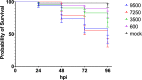
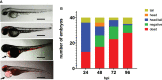
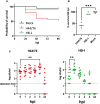
Neisseria meningitidis or the meningococcus, can cause devasting diseases such as sepsis and meningitis. Its polysaccharide capsule, on which serogrouping is based, is the most important virulence factor. Non-encapsulated meningococci only rarely cause disease, due to their sensitivity to the host complement system. How the capsular polysaccharide structure of N. meningitidis relates to virulence is largely unknown. Meningococcal virulence can be modeled in zebrafish embryos as the innate immune system of the zebrafish embryo resembles that of mammals and is fully functional two days post-fertilization. In contrast, the adaptive immune system does not develop before 4 weeks post-fertilization. We generated isogenic meningococcal serogroup variants to study how the chemical composition of the polysaccharide capsule affects N. meningitidis virulence in the zebrafish embryo model. H44/76 serogroup B killed zebrafish embryos in a dose-dependent manner, whereas the non-encapsulated variant was completely avirulent. Neutrophil depletion was observed after infection with encapsulated H44/76, but not with its non-encapsulated variant HB-1. The survival of embryos infected with isogenic capsule variants of H44/76 was capsule specific. The amount of neutrophil depletion differed accordingly. Both embryo killing capacity and neutrophil depletion after infection correlated with the number of carbons used per repeat unit of the capsule polysaccharide during its biosynthesis (indicative of metabolic cost).
Conclusion: Meningococcal virulence in the zebrafish embryo largely depends on the presence of the polysaccharide capsule but the extent of the contribution is determined by its structure. The observed differences between the meningococcal isogenic capsule variants in zebrafish embryo virulence may depend on differences in metabolic cost.
Keywords: Neisseria meningitidis; innate immunity; isogenic capsule variants; meningococcal polysaccharide capsule; zebrafish embryo infection.
Copyright © 2022 Schipper, Preusting, van Sorge, Pannekoek and van der Ende.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abramoff M. D., Magalhaes P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics Int. 11, 36–42.
-
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. (1975). Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups b and c with carbon 13 nuclear magnetic resonance. J. Biol. Chem. 250, 1926–1932. - PubMed
-
- Borrow R., Longworth E., Gray S. J., Kaczmarski E. B. (2000). Prevalence of de-o-acetylated serogroup c meningococci before the introduction of meningococcal serogroup c conjugate vaccines in the united kingdom. FEMS Immunol. Med. Microbiol. 28, 189–191. doi: 10.1111/j.1574-695X.2000.tb01475.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

