Laboratory detection of SARS-CoV-2: A review of the current literature and future perspectives
- PMID: 36212015
- PMCID: PMC9527186
- DOI: 10.1016/j.heliyon.2022.e10858
Laboratory detection of SARS-CoV-2: A review of the current literature and future perspectives
Abstract
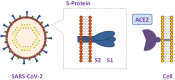
Nowadays, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), whose infectivity is awfully strong, has been a major global threat to the public health. Since lung is the major target of SARS-CoV-2, the infection can lead to respiratory distress syndrome (RDS), multiple organ failure (MOF), and even death. The studies on viral structure and infection mechanism have found that angiotensin-converting enzyme 2 (ACE2), a pivotal enzyme affecting the organ-targeting in the RAS system, is the receptor of the SARS-CoV-2 virus. Currently, the detection of SARSCoV-2 is mainly achieved using open plate real-time reverse-transcription polymerase chain reaction (RT-PCR). While open plate method has some limitations, such as a high false-negative rate, cumbersome manual operation, aerosol pollution and leakage risks. Therefore, a convenient method to rapidly detect SARS-CoV-2 virus is urgently and extremely required for timely epidemic control with the limited resources. In this review, the current real-time methods and principles for novel coronavirus detection are summarized, with the aim to provide a reference for real-time screening of coronavirus in areas with insufficient detection capacity and inadequate medical resources. The development and establishment of a rapid, simple, sensitive and specific system to detect SARS-CoV-2 is of vital importance for distinct diagnosis and effective treatment of the virus, especially in the flu season.
Keywords: Gene editing techniques; Gene sequencing; Novel coronavirus; Nucleic acid testing; SARS-CoV-2.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2).Biosens Bioelectron. 2021 Jan 1;171:112715. doi: 10.1016/j.bios.2020.112715. Epub 2020 Oct 15. Biosens Bioelectron. 2021. PMID: 33099241 Free PMC article.
-
Emergency SARS-CoV-2 Variants of Concern: Novel Multiplex Real-Time RT-PCR Assay for Rapid Detection and Surveillance.Microbiol Spectr. 2022 Feb 23;10(1):e0251321. doi: 10.1128/spectrum.02513-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196812 Free PMC article.
-
[Applications of separation technology in novel coronavirus research, epidemic prevention and detection].Se Pu. 2021 Jul 8;39(7):679-685. doi: 10.3724/SP.J.1123.2021.03022. Se Pu. 2021. PMID: 34227364 Free PMC article. Review. Chinese.
-
Potential effects of COVID-19 on reproductive health: a mini review.Am J Transl Res. 2021 Dec 15;13(12):13321-13327. eCollection 2021. Am J Transl Res. 2021. PMID: 35035678 Free PMC article. Review.
Cited by
-
Machine Learning Techniques for Effective Pathogen Detection Based on Resonant Biosensors.Biosensors (Basel). 2023 Aug 31;13(9):860. doi: 10.3390/bios13090860. Biosensors (Basel). 2023. PMID: 37754094 Free PMC article.
References
-
- Bao Y.F., Xue Q.R., Wu H.P., Zou B., Song Q.X., Zhou G.H. Vol. 51. 2020. Advances in point-of-care Testing for New corona Virus Nucleic acid. J. China Pharm. Univ. pp. 635–645.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

