Light-responsive nanochannels based on the supramolecular host-guest system
- PMID: 36212057
- PMCID: PMC9532542
- DOI: 10.3389/fchem.2022.986908
Light-responsive nanochannels based on the supramolecular host-guest system
Abstract
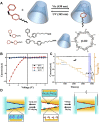
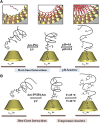
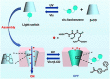
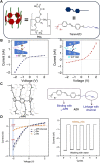
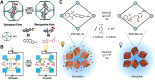
The light-responsive nanochannel of rhodopsin gained wider research interest from its crucial roles in light-induced biological functions, such as visual signal transduction and energy conversion, though its poor stability and susceptibility to inactivation in vitro have limited its exploration. However, the fabrication of artificial nanochannels with the properties of physical stability, controllable structure, and easy functional modification becomes a biomimetic system to study the stimulus-responsive gating properties. Typically, light-responsive molecules of azobenzene (Azo), retinal, and spiropyran were introduced into nanochannels as photo-switches, which can change the inner surface wettability of nanochannels under the influence of light; this ultimately results in the photoresponsive nature of biomimetic nanochannels. Furthermore, the fine-tuning of their stimulus-responsive properties can be achieved through the introduction of host-guest systems generally combined with a non-covalent bond, and the assembling process is reversible. These host-guest systems have been introduced into the nanochannels to form different functions. Based on the host-guest system of light-responsive reversible interaction, it can not only change the internal surface properties of the nanochannel and control the recognition and transmission behaviors but also realize the controlled release of a specific host or guest molecules in the nanochannel. At present, macrocyclic host molecules have been introduced into nanochannels including pillararenes, cyclodextrin (CD), and metal-organic frameworks (MOFs). They are introduced into the nanochannel through chemical modification or host-guest assemble methods. Based on the changes in the light-responsive structure of azobenzene, spiropyran, retinal, and others with macrocycle host molecules, the surface charge and hydrophilic and hydrophobic properties of the nanochannel were changed to regulate the ionic and molecular transport. In this study, the development of photoresponsive host and guest-assembled nanochannel systems from design to application is reviewed, and the research prospects and problems of this photo-responsive nanochannel membrane are presented.
Keywords: functional nanochannels; host–guest system; light response; mass transport; supramolecule.
Copyright © 2022 Quan, Guo, Ma, Long, Wang, Zhang, Sun, Dhinakaran and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Ali M., Nasir S., Ramirez P., Ahmed I., Nguyen Q. H., Fruk L., et al. (2012). Optical gating of photosensitive synthetic ion channels. Adv. Funct. Mat. 22 (2), 390–396. 10.1002/adfm.201102146 - DOI
-
- Arnspang E. C., Sengupta P., Mortensen K. I., Jensen H. H., Hahn U., Jensen E. B. V., et al. (2019). Regulation of plasma membrane nanodomains of the water channel aquaporin-3 revealed by fixed and live photoactivated localization microscopy. Nano Lett. 19 (2), 699–707. 10.1021/acs.nanolett.8b03721 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

