Neoadjuvant immunotherapy and neoadjuvant chemotherapy in resectable non-small cell lung cancer: A systematic review and single-arm meta-analysis
- PMID: 36212419
- PMCID: PMC9533019
- DOI: 10.3389/fonc.2022.901494
Neoadjuvant immunotherapy and neoadjuvant chemotherapy in resectable non-small cell lung cancer: A systematic review and single-arm meta-analysis
Abstract
Background: It remains uncertain whether neoadjuvant immune checkpoint inhibitor (nICI) is superior to neoadjuvant chemotherapy (nCT) in resectable non-small cell lung cancer. In addition, there are outstanding questions for nICI such as the ideal treatment mode and predictors.
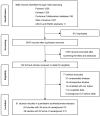
Methods: PubMed, Embase, Cochrane Library, Web of Science, and scientific meetings were searched for eligible single-arm or multi-arm trials until 31 December 2021. The primary outcomes of interest were major pathological response (MPR) and pathological complete response (pCR). The random-effect model was used for statistical analysis.
Results: Twenty-four trials of nICI (n = 1,043) and 29 trials of nCT (n = 2,337) were identified. nICI combination therapy was associated with higher MPR (63.2%, 95% CI: 54.2%-72.1%) and pCR (35.3%, 95% CI: 27.4%-43.3%) rates compared to nCT (16.2%, 95% CI: 7.5%-25.0%, P < 0.001 and 5.5%, 95% CI: 3.5%-7.5%, P < 0.001) and nICI monotherapy (23.3%, 95% CI: 12.7%-33.8%, P < 0.001, and 6.5%, 95% CI: 1.7%-11.2%, P < 0.001). As for safety, nICI monotherapy had the best tolerability; nICI combination showed a similar surgical resection rate and higher R0 resection rate compared to nCT. PD-1 inhibitor and high PD-L1 expression (≥1% or ≥50%) were correlated with higher MPR and pCR rates compared to PD-L1 inhibitor and PD-L1 expression <1%.
Conclusions: nICI combination therapy is associated with higher MPR and pCR rates compared to nCT and nICI monotherapy. PD-1 inhibitor seems to be superior to PD-L1 inhibitor. PD-L1 status appears to be predictive of MPR and pCR for patients receiving nICI.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=278661, CRD42021278661.
Keywords: chemotherapy; immune checkpoint inhibitor; meta-analysis; neoadjuvant; non-small cell lung cancer; pathological response.
Copyright © 2022 Wang, Liu, Chen and Dang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Efficacy and safety of neoadjuvant immunotherapy plus chemotherapy followed by adjuvant immunotherapy in resectable non-small cell lung cancer: a meta-analysis of phase 3 clinical trials.Front Immunol. 2024 Apr 5;15:1359302. doi: 10.3389/fimmu.2024.1359302. eCollection 2024. Front Immunol. 2024. PMID: 38646542 Free PMC article.
-
Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2023 Mar 23;14:1108213. doi: 10.3389/fimmu.2023.1108213. eCollection 2023. Front Immunol. 2023. PMID: 37033991 Free PMC article.
-
Is neoadjuvant immunotherapy necessary in patients with programmed death ligand 1 expression-negative resectable non-small cell lung cancer? A systematic review and meta-analysis.Lung Cancer. 2024 May;191:107799. doi: 10.1016/j.lungcan.2024.107799. Epub 2024 Apr 23. Lung Cancer. 2024. PMID: 38669725
-
Short-term outcome of neoadjuvant immunotherapy and chemotherapy in non-small cell lung cancer: A systematic review and meta-analysis.JTCVS Open. 2021 Sep 2;8:588-607. doi: 10.1016/j.xjon.2021.08.036. eCollection 2021 Dec. JTCVS Open. 2021. PMID: 36004199 Free PMC article.
-
Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2022 Sep 13;13:998620. doi: 10.3389/fimmu.2022.998620. eCollection 2022. Front Immunol. 2022. PMID: 36177019 Free PMC article.
Cited by
-
Clinical Stage III NSCLC Patients Treated with Neoadjuvant Therapy and Surgery: The Prognostic Role of Nodal Characteristics.Life (Basel). 2022 Nov 1;12(11):1753. doi: 10.3390/life12111753. Life (Basel). 2022. PMID: 36362907 Free PMC article.
-
Identifying patients who benefit more from perioperative immunotherapy combinations for resectable non-small cell lung cancer based on clinical and molecular characteristics: a meta-analysis of randomized clinical trials.Clin Transl Oncol. 2025 Apr;27(4):1516-1528. doi: 10.1007/s12094-024-03712-0. Epub 2024 Sep 12. Clin Transl Oncol. 2025. PMID: 39264530
-
What is the ideal endpoint in early-stage immunotherapy neoadjuvant trials in lung cancer?Ther Adv Med Oncol. 2023 Sep 15;15:17588359231198446. doi: 10.1177/17588359231198446. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37720499 Free PMC article. Review.
References
-
- Jair Bar DU, Ofek E, Ackerstein A, Redinsky I, Golan N, Kamer I, et al. . Neoadjuvant pembrolizumab (Pembro) for early stage nonsmall cell lung cancer (NSCLC): updated report of a phase I study, MK3475-223. J Clin Oncol (2019) 37(15_suppl):8534. doi: 10.1200/JCO.2019.37.15_suppl.8534 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

