Cranial autonomic symptoms and response to monoclonal antibodies targeting the Calcitonin gene-related peptide pathway: A real-world study
- PMID: 36212640
- PMCID: PMC9538972
- DOI: 10.3389/fneur.2022.973226
Cranial autonomic symptoms and response to monoclonal antibodies targeting the Calcitonin gene-related peptide pathway: A real-world study
Abstract
Objective: Cranial autonomic symptoms (CAS), including conjunctival injection, tearing, nasal congestion or rhinorrhea, eyelid edema, miosis or ptosis, and forehead or facial sweating ipsilateral to headache, are often reported by patients with migraine during headache attacks. CAS is a consequence of the activation of the trigeminovascular system, which is the target of monoclonal antibodies acting on the CGRP pathway. Therefore, we hypothesized that patients with CAS might have higher trigeminovascular activation than those without CAS leading to a better response to anti-CGRP treatments.
Methods: We performed a prospective analysis including patients with episodic or chronic migraine treated with anti-CGRP monoclonal antibodies (i.e., erenumab, fremanezumab, and galcanezumab) between 2019 and 2021. The observation period included a 12-week baseline before treatment with anti-CGRP antibodies and a 12-week treatment follow-up. We evaluated the prevalence of CAS in our cohort and compared disease characteristics and treatment response (i.e., 12-week monthly headache days and 0-29, 30-49, 50-74, 75-99, and 100% monthly headache days reduction from baseline) among patients with and without CAS using the χ2 test, Kruskal-Wallis test, and Mann-Whitney U-test.
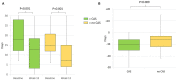
Results: Out of 136 patients, 88 (65%) had CAS. Both patients with and without CAS reported a significant decrease in monthly headache days from baseline. During the 12-week follow-up, the median difference in monthly headache days from baseline was higher in patients with CAS (-10, IQR-15 to-6) than in those without CAS (6, IQR 12 to 3; P = 0.009). However, the proportions of patients with 0 to 29, 30 to 49, 50 to 74, 75 to 99, and 100% response rates did not differ between the two groups.
Conclusions: In our cohort, the presence of CAS was associated with a greater response to monoclonal antibodies targeting the CGRP pathway. CAS could be a clinical marker of trigeminovascular activation and thus be related to a better response to CGRP treatments.
Keywords: CAS; anti-CGRP monoclonal antibodies; cranial autonomic symptoms; migraine; trigeminovascular system.
Copyright © 2022 De Matteis, Caponnetto, Casalena, Frattale, Gabriele, Affaitati, Giamberardino, Maddestra, Viola, Pistoia, Sacco and Ornello.
Conflict of interest statement
Author RO has received sponsorship to attend meetings from Novartis and Teva. Author SS had a financial relationship (lecturer or member of advisory board) with Abbott, Allergan, Novartis, Teva, and Eli Lilly. Author GA has received funds for congress participation from Innovet Italia Srl, Epitech Group, and Lusofarmaco. Author MG received funds for congress participation from IBSA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

