Amphiphilic aminoglycosides: Modifications that revive old natural product antibiotics
- PMID: 36212866
- PMCID: PMC9537547
- DOI: 10.3389/fmicb.2022.1000199
Amphiphilic aminoglycosides: Modifications that revive old natural product antibiotics
Abstract
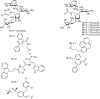
Widely-used Streptomyces-derived antibacterial aminoglycosides have encountered challenges because of antibiotic resistance and toxicity. Today, they are largely relegated to medicinal topical applications. However, chemical modification to amphiphilic aminoglycosides can revive their efficacy against bacterial pathogens and expand their targets to other pathogenic microbes and disorders associated with hyperactive connexin hemichannels. For example, amphiphilic versions of neomycin and neamine are not subject to resistance and have expanded antibacterial spectra, and amphiphilic kanamycins are effective antifungals and have promising therapeutic uses as connexin hemichannel inhibitors. With further research and discoveries aimed at improved formulations and delivery, amphiphilic aminoglycosides may achieve new horizons in pharmacopeia and agriculture for Streptomyces aminoglycosides beyond just serving as topical antibacterials.
Keywords: amphiphilic aminoglycosides; antibiotic resistance; connexin; kanamycin; neamine; neomycin.
Copyright © 2022 Takemoto, Altenberg, Poudyal, Subedi and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


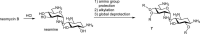



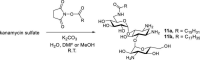

References
-
- Arya D. P. (ed.) (2007). Aminoglycoside Antibiotics: From Chemical Biology to Drug Discovery. Hoboken: John Wiley & Sons, Inc.
Publication types
LinkOut - more resources
Full Text Sources

