Role of the faecal immunochemical test in patients with risk-stratified suspected colorectal cancer symptoms: A systematic review and meta-analysis to inform the ACPGBI/BSG guidelines
- PMID: 36212984
- PMCID: PMC9535300
- DOI: 10.1016/j.lanepe.2022.100518
Role of the faecal immunochemical test in patients with risk-stratified suspected colorectal cancer symptoms: A systematic review and meta-analysis to inform the ACPGBI/BSG guidelines
Abstract
Background: The UK National Institute for Health and Care Excellence (NICE), recommended in 2017 the use of the faecal immunochemical test (FIT) to guide investigations in patients presenting with NICE-defined low-risk symptoms suspicious for colorectal cancer (CRC). At that time, NICE did not recommend FIT use for high-risk symptoms. This is the first systematic review to evaluate the diagnostic accuracy of FIT in NICE-defined high and low-risk symptoms and was designed to inform the joint ACPGBI/BSG guidelines.
Methods: We performed a systematic literature review and meta-analysis. PROSPERO registration number CRD42021224674. Medline and EMBASE databases were searched from inception to 31st March 2022. We included studies recruiting adult patients presenting with suspected CRC symptoms in whom FIT was performed and diagnostic accuracy data for CRC detection could be derived at a limit of detection (LoD) and/or 10 µg haemoglobin/gram faeces threshold in four commonly used analysers. FIT performance was assessed for high-risk, low-risk and individual symptoms where possible. Bivariate meta-analysis was performed where study numbers allowed.
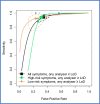
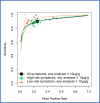
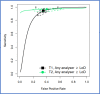
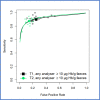
Findings: Thirty-one studies (79566 patients) met inclusion criteria. At 10 µg/g, for "all symptoms" (n = 35,945) sensitivity and specificity were 91.0% (95% CI: 88.9, 92.7) and 75.2% (95% CI: 69.6, 80.1); for "high-risk" symptoms (n = 18,264), 88.7% (95% CI: 84.4, 92.0) and 78.5% (95% CI: 73.0, 83.2); and for "low-risk" symptoms (n = 2161), 88.7% (95% CI: 78.1, 95.3) and 88.5% (95% CI: 87.1, 89.9), respectively. At LoD, for "all symptoms" (n = 26,056) sensitivity and specificity were 94.7% (95% CI: 90.5, 97.1) and 66.5% (95% CI: 58.7, 73.6); for "high-risk" symptoms (n = 16,768), 92.8% (95% CI: 86.4, 96.3) and 70.3% (95% CI: 66.5, 73.8); and for "low-risk" symptoms (n = 2082), 94.7% (95% CI: 85.4, 98.9) and 71.9% (95% CI: 69.9, 73.9), respectively. Summary estimates were similar across different analysers.
Interpretation: FIT sensitivity for CRC detection is maximised at the LoD; its performance is similar in high and low-risk symptoms, and across different analysers where a common threshold is used. FIT performance for CRC detection is adequate and transferrable to clinical diagnostic pathways.
Funding: This review was part-funded by NHS England awarded to RM Partners. RB and RC were funded by research fellowships awarded by Croydon University Hospital.
Keywords: Clinical decision making; Colorectal cancer; FOB Gold; Faecal immunochemical test, FIT; HM-JACKarc; High-risk symptoms; Low-risk symptoms; Meta-analysis; NICE DG30; NICE NG12; OC-Sensor; QuikRead go; Stool markers; Systematic review.
© 2022 The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures

References
-
- Cancer Research UK, https://www.cancerresearchuk.org/health-professional/cancer-statistics/s..., Accessed November 2021
-
- Cancer Research UK, https://crukcancerintelligence.shinyapps.io/EarlyDiagnosis/, Accessed November 2021
-
- NICE . National Institute for Health and Care Excellence; 2005. Referral Guidelines for Suspected Cancer [CG27]
-
- NICE . National Institute for Health and Care Excellence; 2015. Suspected Cancer: Recognition and Referral [NG12] - PubMed
-
- NICE . National Institute for Health and Care Excellence; 2017. Diagnostics Guidance [DG30]. Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

