Neural mechanisms for turn-taking in duetting plain-tailed wrens
- PMID: 36213202
- PMCID: PMC9537813
- DOI: 10.3389/fncir.2022.970434
Neural mechanisms for turn-taking in duetting plain-tailed wrens
Erratum in
-
Corrigendum: Neural mechanisms for turn-taking in duetting plain-tailed wrens.Front Neural Circuits. 2022 Dec 8;16:1068385. doi: 10.3389/fncir.2022.1068385. eCollection 2022. Front Neural Circuits. 2022. PMID: 36569800 Free PMC article.
Abstract
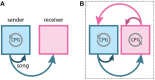
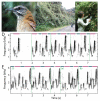
Recent studies conducted in the natural habitats of songbirds have provided new insights into the neural mechanisms of turn-taking. For example, female and male plain-tailed wrens (Pheugopedius euophrys) sing a duet that is so precisely timed it sounds as if a single bird is singing. In this review, we discuss our studies examining the sensory and motor cues that pairs of wrens use to coordinate the rapid alternation of syllable production. Our studies included behavioral measurements of freely-behaving wrens in their natural habitat and neurophysiological experiments conducted in awake and anesthetized individuals at field sites in Ecuador. These studies show that each partner has a pattern-generating circuit in their brain that is linked via acoustic feedback between individuals. A similar control strategy has been described in another species of duetting songbird, white-browed sparrow-weavers (Plocepasser mahali). Interestingly, the combination of neurophysiological results from urethane-anesthetized and awake wrens suggest a role for inhibition in coordinating the timing of turn-taking. Finally, we highlight some of the unique challenges of conducting these experiments at remote field sites.
Keywords: antiphonal; auditory feedback; birdsong; central pattern generator; duets; neuroethology.
Copyright © 2022 Coleman, Day and Fortune.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Accorsi-Mendonça D., Leão R. M., Aguiar J. F., Varanda W. A., Machado B. H. (2007). Urethane inhibits the GABAergic neurotransmission in the nucleus of the solitary tract of rat brain stem slices. Am. J. Physiol. Regulat. Integrat. Compar. Physiol. 292, R396–R402. 10.1152/ajpregu.00776.2005 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

