An implantable neurophysiology platform: Broadening research capabilities in free-living and non-traditional animals
- PMID: 36213207
- PMCID: PMC9537467
- DOI: 10.3389/fncir.2022.940989
An implantable neurophysiology platform: Broadening research capabilities in free-living and non-traditional animals
Abstract
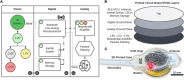
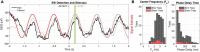
Animal-borne sensors that can record and transmit data ("biologgers") are becoming smaller and more capable at a rapid pace. Biologgers have provided enormous insight into the covert lives of many free-ranging animals by characterizing behavioral motifs, estimating energy expenditure, and tracking movement over vast distances, thereby serving both scientific and conservational endpoints. However, given that biologgers are usually attached externally, access to the brain and neurophysiological data has been largely unexplored outside of the laboratory, limiting our understanding of how the brain adapts to, interacts with, or addresses challenges of the natural world. For example, there are only a handful of studies in free-living animals examining the role of sleep, resulting in a wake-centric view of behavior despite the fact that sleep often encompasses a large portion of an animal's day and plays a vital role in maintaining homeostasis. The growing need to understand sleep from a mechanistic viewpoint and probe its function led us to design an implantable neurophysiology platform that can record brain activity and inertial data, while utilizing a wireless link to enable a suite of forward-looking capabilities. Here, we describe our design approach and demonstrate our device's capability in a standard laboratory rat as well as a captive fox squirrel. We also discuss the methodological and ethical implications of deploying this new class of device "into the wild" to fill outstanding knowledge gaps.
Keywords: accelerometer; closed-loop; implantable; physiology; sleep; wireless.
Copyright © 2022 Gaidica and Dantzer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


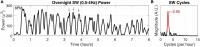
References
-
- Bakker J., Klomp R., Rijnbeek M. W., Arndt S. S., Philippens I. H., Langermans J. A. (2014). Recovery time after intra-abdominal transmitter placement for telemetric (neuro) physiological measurement in freely moving common marmosets (Callitrix jacchus). Anim. Biotelem. 2:10. 10.1186/2050-3385-2-10 - DOI
-
- Barron D. G., Brawn J. D., Weatherhead P. J. (2010). Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1 180–187. 10.1111/j.2041-210X.2010.00013.x - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

