The global landscape of approved antibody therapies
- PMID: 36213257
- PMCID: PMC9535261
- DOI: 10.1093/abt/tbac021
The global landscape of approved antibody therapies
Abstract
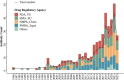
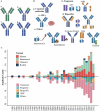
Antibody therapies have become an important class of therapeutics in recent years as they have exhibited outstanding efficacy and safety in the treatment of several major diseases including cancers, immune-related diseases, infectious disease and hematological disease. There has been significant progress in the global research and development landscape of antibody therapies in the past decade. In this review, we have collected available data from the Umabs Antibody Therapies Database (Umabs-DB, https://umabs.com) as of 30 June 2022. The Umabs-DB shows that 162 antibody therapies have been approved by at least one regulatory agency in the world, including 122 approvals in the US, followed by 114 in Europe, 82 in Japan and 73 in China, whereas biosimilar, diagnostic and veterinary antibodies are not included in our statistics. Although the US and Europe have been at the leading position for decades, rapid advancement has been witnessed in Japan and China in the past decade. The approved antibody therapies include 115 canonical antibodies, 14 antibody-drug conjugates, 7 bispecific antibodies, 8 antibody fragments, 3 radiolabeled antibodies, 1 antibody-conjugate immunotoxin, 2 immunoconjugates and 12 Fc-Fusion proteins. They have been developed against 91 drug targets, of which PD-1 is the most popular, with 14 approved antibody-based blockades for cancer treatment in the world. This review outlined the global landscape of the approved antibody therapies with respect to the regulation agencies, therapeutic targets and indications, aiming to provide an insight into the trends of the global development of antibody therapies.
Keywords: antibody format; antibody targets; antibody therapies; approved antibody; global regulatory agency.
© The Author(s) 2022. Published by Oxford University Press on behalf of Antibody Therapeutics. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- NCI dictionary of Cancer Terms . In: National Cancer Institute. 2 Feb 2011. [cited 1 Jul 2022]. Available: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/antibo...
-
- Van Wauwe, JP, De Mey, JR, Goossens, JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol 1980; 124: 2708–13. - PubMed
-
- Reichert, JM, Valge-Archer, VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov 2007; 6: 349–56. - PubMed
-
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov 2021; 20: 491–5. - PubMed
-
- Edvinsson, L, Haanes, KA, Warfvinge, Ket al. . CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 2018; 14: 338–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
