Improving risk indexes for Alzheimer's disease and related dementias for use in midlife
- PMID: 36213312
- PMCID: PMC9535507
- DOI: 10.1093/braincomms/fcac223
Improving risk indexes for Alzheimer's disease and related dementias for use in midlife
Abstract
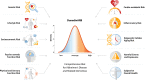
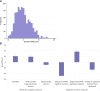
Knowledge of a person's risk for Alzheimer's disease and related dementias (ADRDs) is required to triage candidates for preventive interventions, surveillance, and treatment trials. ADRD risk indexes exist for this purpose, but each includes only a subset of known risk factors. Information missing from published indexes could improve risk prediction. In the Dunedin Study of a population-representative New Zealand-based birth cohort followed to midlife (N = 938, 49.5% female), we compared associations of four leading risk indexes with midlife antecedents of ADRD against a novel benchmark index comprised of nearly all known ADRD risk factors, the Dunedin ADRD Risk Benchmark (DunedinARB). Existing indexes included the Cardiovascular Risk Factors, Aging, and Dementia index (CAIDE), LIfestyle for BRAin health index (LIBRA), Australian National University Alzheimer's Disease Risk Index (ANU-ADRI), and risks selected by the Lancet Commission on Dementia. The Dunedin benchmark was comprised of 48 separate indicators of risk organized into 10 conceptually distinct risk domains. Midlife antecedents of ADRD treated as outcome measures included age-45 measures of brain structural integrity [magnetic resonance imaging-assessed: (i) machine-learning-algorithm-estimated brain age, (ii) log-transformed volume of white matter hyperintensities, and (iii) mean grey matter volume of the hippocampus] and measures of brain functional integrity [(i) objective cognitive function assessed via the Wechsler Adult Intelligence Scale-IV, (ii) subjective problems in everyday cognitive function, and (iii) objective cognitive decline measured as residualized change in cognitive scores from childhood to midlife on matched Weschler Intelligence scales]. All indexes were quantitatively distributed and proved informative about midlife antecedents of ADRD, including algorithm-estimated brain age (β's from 0.16 to 0.22), white matter hyperintensities volume (β's from 0.16 to 0.19), hippocampal volume (β's from -0.08 to -0.11), tested cognitive deficits (β's from -0.36 to -0.49), everyday cognitive problems (β's from 0.14 to 0.38), and longitudinal cognitive decline (β's from -0.18 to -0.26). Existing indexes compared favourably to the comprehensive benchmark in their association with the brain structural integrity measures but were outperformed in their association with the functional integrity measures, particularly subjective cognitive problems and tested cognitive decline. Results indicated that existing indexes could be improved with targeted additions, particularly of measures assessing socioeconomic status, physical and sensory function, epigenetic aging, and subjective overall health. Existing premorbid ADRD risk indexes perform well in identifying linear gradients of risk among members of the general population at midlife, even when they include only a small subset of potential risk factors. They could be improved, however, with targeted additions to more holistically capture the different facets of risk for this multiply determined, age-related disease.
Keywords: Alzheimer’s disease; dementia; modifiable risk factors; preventive medicine; risk index.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
Dementia, dementia's risk factors and premorbid brain structure are concentrated in disadvantaged areas: National register and birth-cohort geographic analyses.Alzheimers Dement. 2024 May;20(5):3167-3178. doi: 10.1002/alz.13727. Epub 2024 Mar 14. Alzheimers Dement. 2024. PMID: 38482967 Free PMC article.
-
Measures of retinal health successfully capture risk for Alzheimer's disease and related dementias at midlife.J Alzheimers Dis. 2025 Mar 3:13872877251321114. doi: 10.1177/13872877251321114. Online ahead of print. J Alzheimers Dis. 2025. PMID: 40033783 Free PMC article.
-
Association of Childhood Lead Exposure With MRI Measurements of Structural Brain Integrity in Midlife.JAMA. 2020 Nov 17;324(19):1970-1979. doi: 10.1001/jama.2020.19998. JAMA. 2020. PMID: 33201203 Free PMC article.
-
Dementia -- Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review [Internet].Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. PMID: 28876770 Free Books & Documents. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Social isolation from childhood to mid-adulthood: is there an association with older brain age?Psychol Med. 2023 Dec;53(16):7874-7882. doi: 10.1017/S0033291723001964. Epub 2023 Jul 24. Psychol Med. 2023. PMID: 37485695 Free PMC article.
-
Cardiovascular risk factors among older persons with cognitive frailty in middle income country.World J Clin Cases. 2024 Jun 16;12(17):3076-3085. doi: 10.12998/wjcc.v12.i17.3076. World J Clin Cases. 2024. PMID: 38898873 Free PMC article.
-
Brain structure and connectivity mediate the association between lifestyle and cognition: The Maastricht Study.Brain Commun. 2024 May 16;6(3):fcae171. doi: 10.1093/braincomms/fcae171. eCollection 2024. Brain Commun. 2024. PMID: 38846531 Free PMC article.
-
Dementia, dementia's risk factors and premorbid brain structure are concentrated in disadvantaged areas: National register and birth-cohort geographic analyses.Alzheimers Dement. 2024 May;20(5):3167-3178. doi: 10.1002/alz.13727. Epub 2024 Mar 14. Alzheimers Dement. 2024. PMID: 38482967 Free PMC article.
-
Measures of retinal health successfully capture risk for Alzheimer's disease and related dementias at midlife.J Alzheimers Dis. 2025 Mar 3:13872877251321114. doi: 10.1177/13872877251321114. Online ahead of print. J Alzheimers Dis. 2025. PMID: 40033783 Free PMC article.
References
-
- Alzheimer’s Disease International . World Alzheimer Report 2019: Attitudes to dementia. 2019. Accessed 16 February 2021. https://www.alzint.org/resource/world-alzheimer-report-2019/.
-
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. - PubMed
-
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Committee on Preventing Dementia and Cognitive Impairment . Preventing cognitive decline and dementia: A way forward. Downey A, Stroud C, Landis S, Leshner AI, eds. National Academies Press; 2017. Accessed 16 February 2021. http://www.ncbi.nlm.nih.gov/books/NBK436397/. - PubMed
-
- Musiek ES, Morris JC. Possible consequences of the approval of a disease-modifying therapy for Alzheimer disease. JAMA Neurol. 2021;78(2):141–142. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
