Permissive Cardiotoxicity: The Clinical Crucible of Cardio-Oncology
- PMID: 36213359
- PMCID: PMC9537074
- DOI: 10.1016/j.jaccao.2022.07.005
Permissive Cardiotoxicity: The Clinical Crucible of Cardio-Oncology
Abstract
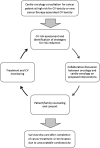
The field of cardio-oncology was born from the necessity for recognition and management of cardiovascular diseases among patients with cancer. This need for this specialty continues to grow as patients with cancer live longer as a result of lifesaving targeted and immunologic cancer therapies beyond the usual chemotherapy and/or radiation therapy. Often, potentially cardiotoxic anticancer treatment is necessary in patients with baseline cardiovascular disease. Moreover, patients may need to continue therapy in the setting of incident cancer therapy-associated cardiotoxicity. Herein, we present and discuss the concept of permissive cardiotoxicity as a novel term that represents an essential concept in the field of cardio-oncology and among practicing cardio-oncology specialists. It emphasizes a proactive rather than reactive approach to continuation of lifesaving cancer therapies in order to achieve the best oncologic outcome while mitigating associated and potentially off-target cardiotoxicities.
Keywords: 5-FU, 5-fluorouracil; CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease; GDMT, guideline-directed medical therapy; GLS, global longitudinal strain; HER2 therapy; HER2, human epidermal growth factor receptor 2; HSCT, hematopoietic stem cell transplantation; ICI, immune checkpoint inhibitor; LVEF, left ventricular ejection fraction; VEGF, vascular endothelial growth factor; cardiomyopathy; diagnosis; immunotherapy; prevention; risk factor; risk prediction; screening; treatment planning.
© 2022 The Authors.
Conflict of interest statement
Dr Dent is a consultant to and has received honoraria from AstraZeneca and Novartis. Dr Lenihan is a consultant (modest) to AstraZeneca, Bristol Myers Squibb, Myocardial Solutions, Clementia, Intellia, Bridge Bio, OncXerna, and SecuraBio. Dr Okwuosa is a consultant to Antev. Dr Porter has received an unrestricted grant from the Sally and Cloud Cray Family Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. - PubMed
-
- Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

