Scribble basal polarity acquisition in RPE cells and its mislocalization in a pathological AMD-like model
- PMID: 36213611
- PMCID: PMC9539273
- DOI: 10.3389/fnana.2022.983151
Scribble basal polarity acquisition in RPE cells and its mislocalization in a pathological AMD-like model
Abstract
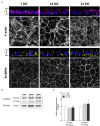
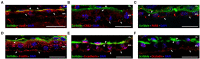
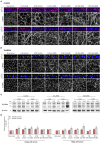
Apicobasal polarity is a hallmark of retinal pigment epithelium cells and is required to perform their functions; however, the precise roles of the different proteins that execute polarity are still poorly understood. Here, we have studied the expression and location of Scribble, the core member of the polarity basal protein complex in epithelial-derived cells, in human and mouse RPE cells in both control and pathological conditions. We found that Scribble specifically localizes at the basolateral membrane of mouse and human RPE cells. In addition, we observed an increase in the expression of Scribble during human RPE development in culture, while it acquires a well-defined basolateral pattern as this process is completed. Finally, the expression and location of Scribble were analyzed in human RPE cells in experimental conditions that mimic the toxic environment suffered by these cells during AMD development and found an increase in Scribble expression in cells that develop a pathological phenotype, suggesting that the protein could be altered in cells under stress conditions, as occurs in AMD. Together, our results demonstrate, for the first time, that Scribble is expressed in both human and mouse RPE and is localized at the basolateral membrane in mature cells. Furthermore, Scribble shows impaired expression and location in RPE cells in pathological conditions, suggesting a possible role for this protein in the development of pathologies, such as AMD.
Keywords: Scribble; Scribble complex; age-related macular degeneration (AMD); cell polarity; differentiation; epithelium; retina; retinal pigment epithelium (RPE).
Copyright © 2022 Segurado, Rodríguez-Carrillo, Castellanos, Hernández-Galilea, Velasco and Lillo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ach T., Tolstik E., Messinger J. D., Zarubina A. V., Heintzmann R., Curcio C. A. (2015). Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest. Ophthalmol. Visual Sci. 56, 3242–3252. 10.1167/iovs.14-16274 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

