Clinical profile in KMT2A-SEPT6- positive acute myeloid leukemia: Does it often co-occur with NRAS mutations?
- PMID: 36213638
- PMCID: PMC9532577
- DOI: 10.3389/fmed.2022.890959
Clinical profile in KMT2A-SEPT6- positive acute myeloid leukemia: Does it often co-occur with NRAS mutations?
Abstract
Background: The KMT2A-SEPT6 fusion gene is a relatively rare genetic event in leukemia. Its clinical characteristics and prognosis, especially the profile of co-occurring gene mutations remain unclear.
Methods: We retrospectively analyzed the characteristics of four cases carrying KMT2A-SEPT6 in our hospital, and provided a literature review.
Results: All the four patients were diagnosed with acute myeloid leukemia (AML) and harbored X chromosome and 11 chromosome rearrangements, they all manifested high levels of D-dimer. Three of four patients had NRAS mutations while one patient with congenital AML did not. Of the four cases, one developed drug resistance, one suffered relapse after bone marrow transplantation (BMT) and two died. Combined with other cases reported in the literature, we found that of all patients diagnosed with AML, 90.9% were children (≤9 years old). Patients with white blood cells ≥20.0 × 109/L or diagnosed with M4 had a shorter overall survival (P < 0.05). Age, whether to receive BMT, and the chromosome rearrangement patterns had no significant effect on overall survival (P > 0.05).
Conclusions: KMT2A-SEPT6 was more commonly observed in pediatric AML patients, some of which may co-occur with NRAS mutations. The prognosis was related to the white blood cell levels and the leukemia subtype, but was not related to age or BMT. More cases need to be accumulated to better understand the profile in KMT2A-SEPT6-positive AML.
Keywords: KMT2A-SEPT6; NRAS; acute myeloid leukemia; gene rearrangement; mutations.
Copyright © 2022 Chen, Yang and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
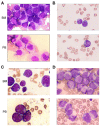
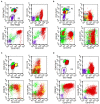

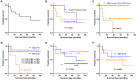
References
-
- Wander P, Arentsen-Peters STCJM, Pinhan?os SS, Koopmans B, Dolman MEM, Ariese R, et al. High-throughput drug screening reveals Pyrvinium pamoate as effective candidate against pediatric MLL-rearranged acute myeloid leukemia. Transl Oncol. (2021) 14:101048. 10.1016/j.tranon.2021.101048 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

