Case report: Treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence
- PMID: 36213654
- PMCID: PMC9537824
- DOI: 10.3389/fmed.2022.1003103
Case report: Treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence
Abstract
Introduction: Long COVID, or post-acute sequelae of SARS-CoV-2 infection (PASC) in ∼30% of all infected individuals. Here, we present a case of PASC in a patient with rheumatoid arthritis characterized by viral persistence in the nasopharynx for 6 months after acute infection. We demonstrate transient disappearance of antigen persistence and decreased antiviral and autoimmune T cell responses after nirmatrelvir/ritonavir and tocilizumab treatment.
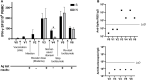
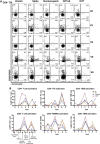
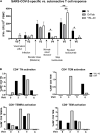
Case presentation: A 37-year-old female with a 7-year history of rheumatoid arthritis enrolled in a COVID-19 research study was found to continuously test SARS-CoV-2 antigen positive in the nasopharynx for 6 months after acute infection. She simultaneously presented with new-onset PASC symptoms including chronic occipital headache and periods of intense fatigue 8 weeks after acute infection. The patient was prescribed nirmatrelvir/ritonavir to treat SARS-CoV-2 persistence at 3.5 months post-acute infection and observed a reduction in PASC symptoms 3 weeks after completing antiviral treatment. After resurgence of PASC symptoms, she stopped treatment with tocilizumab for rheumatoid arthritis to attempt complete SARS-CoV-2 viral clearance. The severity of the patient's PASC symptoms subsequently increased, and she developed new-onset brain fog in addition to previous symptoms, which resolved after resumption of tocilizumab treatment. Assessment of adaptive immune responses demonstrated that nirmatrelvir/ritonavir and tocilizumab treatment decreased antiviral and autoreactive T cell activation. After resuming tocilizumab treatment, the patient's PASC symptoms were significantly reduced, but nasopharyngeal antigen positivity remained.
Conclusion: These data suggest that nirmatrelvir/ritonavir should be considered in the treatment of PASC in patients who have SARS-CoV-2 antigen persistence, though care must be taken to monitor the patient for symptom resurgence or viral reactivation. In addition, the IL-6 inhibitor tocilizumab may ameliorate PASC symptoms in patients with persistent headache, fatigue, and brain fog.
Keywords: autoimmunity; case report; long COVID; nirmatrelvir/ritonavir; tocilizumab.
Copyright © 2022 Visvabharathy, Orban and Koralnik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae.BMC Infect Dis. 2021 Jul 13;21(1):677. doi: 10.1186/s12879-021-06359-2. BMC Infect Dis. 2021. PMID: 34256735 Free PMC article.
-
Prevalence and determinants of post-acute sequelae after SARS-CoV-2 infection (Long COVID) among adults in Mexico during 2022: a retrospective analysis of nationally representative data.Lancet Reg Health Am. 2024 Feb 3;30:100688. doi: 10.1016/j.lana.2024.100688. eCollection 2024 Feb. Lancet Reg Health Am. 2024. PMID: 38327277 Free PMC article.
-
Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment.Clin Infect Dis. 2023 Feb 18;76(4):573-581. doi: 10.1093/cid/ciac663. Clin Infect Dis. 2023. PMID: 36200701 Free PMC article.
-
Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) and the Urgent Need to Identify Diagnostic Biomarkers and Risk Factors.Med Sci Monit. 2024 Sep 18;30:e946512. doi: 10.12659/MSM.946512. Med Sci Monit. 2024. PMID: 39289865 Free PMC article. Review.
-
Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC).Elife. 2023 Mar 22;12:e86002. doi: 10.7554/eLife.86002. Elife. 2023. PMID: 36947108 Free PMC article. Review.
Cited by
-
Mechanisms of long COVID and the path toward therapeutics.Cell. 2024 Oct 3;187(20):5500-5529. doi: 10.1016/j.cell.2024.07.054. Epub 2024 Sep 25. Cell. 2024. PMID: 39326415 Review.
-
EFFECT OF PAXLOVID TREATMENT DURING ACUTE COVID-19 ON LONG COVID ONSET: AN EHR-BASED TARGET TRIAL EMULATION FROM THE N3C AND RECOVER CONSORTIA.medRxiv [Preprint]. 2025 Apr 7:2024.01.20.24301525. doi: 10.1101/2024.01.20.24301525. medRxiv. 2025. PMID: 38343863 Free PMC article. Preprint.
-
Detectable plasma severe acute respiratory syndrome coronavirus 2 spike antigen is associated with poor antibody response following third messenger RNA vaccination in kidney transplant recipients.Transpl Infect Dis. 2024 Jun;26(3):e14281. doi: 10.1111/tid.14281. Epub 2024 Apr 15. Transpl Infect Dis. 2024. PMID: 38618895 Free PMC article.
-
Plasma proteomics show altered inflammatory and mitochondrial proteins in patients with neurologic symptoms of post-acute sequelae of SARS-CoV-2 infection.Brain Behav Immun. 2023 Nov;114:462-474. doi: 10.1016/j.bbi.2023.08.022. Epub 2023 Sep 11. Brain Behav Immun. 2023. PMID: 37704012 Free PMC article.
-
Combining Double-Dose and High-Dose Pulsed Dapsone Combination Therapy for Chronic Lyme Disease/Post-Treatment Lyme Disease Syndrome and Co-Infections, Including Bartonella: A Report of 3 Cases and a Literature Review.Microorganisms. 2024 Apr 30;12(5):909. doi: 10.3390/microorganisms12050909. Microorganisms. 2024. PMID: 38792737 Free PMC article.
References
-
- Johns Hopkins Coronavirus Resource Center. Cumulative Worldwide Covid-19 Cases. Baltimore, MD: Johns Hopkins University of Medicine; (2021).
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

