Cholecystectomy promotes the development of colorectal cancer by the alternation of bile acid metabolism and the gut microbiota
- PMID: 36213655
- PMCID: PMC9540502
- DOI: 10.3389/fmed.2022.1000563
Cholecystectomy promotes the development of colorectal cancer by the alternation of bile acid metabolism and the gut microbiota
Abstract
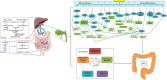
The incidence and mortality of colorectal cancer (CRC) have been markedly increasing worldwide, causing a tremendous burden to the healthcare system. Therefore, it is crucial to investigate the risk factors and pathogenesis of CRC. Cholecystectomy is a gold standard procedure for treating symptomatic cholelithiasis and gallstone diseases. The rhythm of bile acids entering the intestine is altered after cholecystectomy, which leads to metabolic disorders. Nonetheless, emerging evidence suggests that cholecystectomy might be associated with the development of CRC. It has been reported that alterations in bile acid metabolism and gut microbiota are the two main reasons. However, the potential mechanisms still need to be elucidated. In this review, we mainly discussed how bile acid metabolism, gut microbiota, and the interaction between the two factors influence the development of CRC. Subsequently, we summarized the underlying mechanisms of the alterations in bile acid metabolism after cholecystectomy including cellular level, molecular level, and signaling pathways. The potential mechanisms of the alterations on gut microbiota contain an imbalance of bile acid metabolism, cellular immune abnormality, acid-base imbalance, activation of cancer-related pathways, and induction of toxin, inflammation, and oxidative stress.
Keywords: bile acid metabolism; cholecystectomy; colorectal cancer; development; gut microbiota.
Copyright © 2022 Jiang, Jiang, Cheng, Sun, Jiang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

