Motor Responses in Pediatric Pompe Disease in the ADVANCE Participant Cohort
- PMID: 36214004
- PMCID: PMC9697057
- DOI: 10.3233/JND-210784
Motor Responses in Pediatric Pompe Disease in the ADVANCE Participant Cohort
Abstract
Background: ADVANCE (NCT01526785) presented an opportunity to obtain a more nuanced understanding of motor function changes in treatment-experienced children with Pompe disease receiving 4000L-production-scale alglucosidase alfa for 52 weeks.
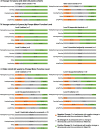
Objective: To estimate minimal detectable change (MDC) and effect size on Gross Motor Function Measure-88 (GMFM-88) after 52 weeks of 4000L alglucosidase alfa (complete data N = 90).
Methods: The GMFM-88 mean total % score changes, MDC, and effect size were analyzed post hoc by Pompe Motor Function Level at enrollment, age groups at enrollment, and fraction of life on pre-study 160L-production-scale alglucosidase alfa.
Results: Overall, participants aged < 2 years surpassed MDC at Week 52 (change [mean±standard deviation] 21.1±14.1, MDC range 5.7-13.3, effect size 1.1), whereas participants aged≥2 years did not attain this (change -0.9±15.3, MDC range 10.8-25.2, effect size -0.03). In participants aged < 2 years, improvements surpassed the MDC for walkers (change 17.1±13.3, MDC range 3.0-6.9, effect size 1.7), supported standers (change 35.2±18.0, MDC range 5.9-13.7, effect size 1.8) and sitters (change 24.1±12.1, MDC range 2.6-6.2, effect size 2.7). Age-independent MDC ranges were only attained by walkers (change 7.7±12.3, MDC range 6.4-15.0, effect size 0.4) and sitters (change 9.9±17.2, MDC range 3.3-7.7, effect size 0.9).
Conclusions: These first GMFM-88 minimal-detectable-change estimates for alglucosidase alfa-treated Pompe disease offer utility for monitoring motor skills.
Trial registration: ClinicalTrials.gov; NCT01526785; Registered 6 February 2012; https://clinicaltrials.gov/ct2/show/NCT01526785.
Keywords: Alglucosidase alfa; Gross Motor Function Classification System; Gross Motor Function Measure-88; Pompe Motor Function History; infantile-onset Pompe disease; late-onset Pompe disease; minimal detectable change.
Conflict of interest statement
TD reports Pompe-disease-related honoraria from Sanofi (advisory boards within and outside the scope of the study), membership of advisory boards for Roche, Scholar Rock, Cure SMA, Cytokinetics, Biogen, Novartis, Bristol Myers Squibb, and SMA Foundation, and service as a consultant for Roche, Audentes, Bristol Myers Squibb, Novartis, Trinds, ATOM International, and Scholar Rock. PSK reports grants from Amicus Therapeutics, Sanofi, and Valerion Therapeutics; consulting fees and honoraria from Amicus Therapeutics, Asklepios BioPharmaceuticals (AskBio), and Sanofi; membership of the Pompe and Gaucher Disease Registry Advisory Boards for Amicus Therapeutics, Baebies, and Sanofi; and equity with Asklepios BioPharmaceuticals (AskBio). JBG discloses study sponsorship and professional writing assistance from Sanofi during the conduct of the study and advisory board membership for Mallinckrodt Pharmaceuticals outside the submitted work. SHH reports personal fees from Alexion, employment by Seattle Children’s Hospital, and personal fees and grants from Sanofi outside the submitted work. RH has nothing to disclose. DK reports research funding from Sanofi and New York Medical College during the conduct of the study and is on the Sanofi speakers’ bureau for Pompe disease. NDL reports personal fees (honoraria for advisory board activities) and non-financial support (professional writing support) from Sanofi during the conduct of the study and outside the submitted work. LDMP discloses grant support and advisory board membership for AveXis, grant support from Biogen, Ionis, and Sanofi, advisory board membership for Roche/Genentech, and research support from Roivant, Inc. DWS is a member of the Sanofi Pompe Registry Advisory Board outside the submitted work and discloses grant support from Sanofi during the conduct of the study. PT discloses current employment by Quest Diagnostics. KAH, SS, and MCF disclose being employees of Sanofi and may hold stock and/or stock options in the company; CW discloses a contract with Sanofi for consulting work during the conduct of the study and services as a consultant for REGENXBIO. JWD reports personal fees from Audentes, grants from Ionis Pharmaceuticals, and grants and personal fees from AveXis, Biogen, Cytokinetics, Roche/Genentech, Sarepta, and Scholar Rock, outside the submitted work; he also has a patent 7442782 with royalties paid to Athena Diagnostics.
Figures
References
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

