Translational value of preclinical models for renal denervation: a histological comparison of human versus porcine renal nerve anatomy
- PMID: 36214318
- PMCID: PMC9909452
- DOI: 10.4244/EIJ-D-22-00369
Translational value of preclinical models for renal denervation: a histological comparison of human versus porcine renal nerve anatomy
Abstract
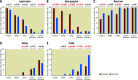
Background: Preclinical models have provided key insights into the response of local tissues to radiofrequency (RF) renal denervation (RDN) that is unobtainable from human studies. However, the anatomic translatability of these models to the procedure in humans is incompletely understood. Aims: We aimed to compare the renal arterial anatomy in normotensive pigs treated with RF-RDN to that of human cadavers to evaluate the suitability of normotensive pigs for determining the safety of RF-RDN.
Methods: Histopathologic analyses were performed on RF-treated renal arteries in a porcine model and untreated control renal arteries. Similar analyses were performed on untreated renal arteries from human cadavers. Results: In both human and porcine renal arteries, the median number of nerves was lower in the more distal sections (the numbers in the proximal, middle, distal, 1st bifurcation, and 2nd bifurcation sections were 65, 58, 47, 22.5, and 14.7 in humans, respectively, and 39, 26, 29, 16.5, and 9.3 in the porcine models, respectively). Renal nerves were common in the regions between arteries and adjacent veins, but only 3% and 13% of the renal nerves in humans and pigs, respectively, were located behind the renal vein. The semiquantitative score of RF-induced renal arterial nerve necrosis was significantly greater at 7 days than 28 days (0.98 vs 0.75; p=0.01), and injury to surrounding organs was rarely observed.
Conclusions: The distribution of nerve tissue and the relative distribution of extravascular anatomic structures along the renal artery was similar between humans and pigs, which validates the translational value of the normotensive porcine model for RDN.
Conflict of interest statement
A. Sharp receives consulting fees/honoraria from Medtronic, Philips and ReCor Medical. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie and Deutsche Forschungsgemeinschaft (SFB 219); and has received scientific support and/or speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, Merck, and ReCor Medical. S. Tunev is a full-time employee of Medtronic. J. Trudel is a full-time employee of Medtronic. D.A. Hettrick is a full-time employee of Medtronic. M. Schlaich is supported by an NHMRC Senior Research Fellowship; and has received consulting fees and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim. D. Lee reports grants from and serves on the advisory board for Medtronic; and research support from Ablative Solutions. D.E. Kandzari reports institutional research/grant support from Medtronic CardioVascular and Ablative Solutions and personal consulting honoraria from Medtronic CardioVascular and Ablative Solutions. A.V. Finn reports consulting honoraria from Amgen, Abbott Vascular, Biosensors, Boston Scientific, CeloNova, Cook Medical, CSI, Lutonix Bard, Medtronic, and Terumo. R. Virmani reports institutional grant/research support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, AerWave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon USA, Cardiac Implants, Cardiawave, CardioMech, Cardionomic, CeloNova, Cerus EndoVascular, Chansu Vascular Technologies, Childrens National Medical Center, Concept Medical, Cook Medical, Cooper Health, Cormaze Technologies GmbH, CRL/AccelLab, Croivalve, CSI, Dexcom, Edwards Lifesciences, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative Cardiovascular Solutions, Intact Vascular, Interface Biologics, InterShunt Technologies, InVatin Technologies, Lahav CRO, LimFlow, L&J Biosciences, Lutonix, Lyra Therapeutics, Mayo Clinic, Maywell, MD Start, MedAlliance, Medanex, Medtronic, Mercator, Microport, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occultech, Olympus, Ohio Health, OrbusNeich, Ossio, Phenox, Pi-Cardia, Polares Medical, Polyvascular, PulseTherapeutics, Profusa, ProKidney, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, ReCor Medical, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, Sahajanand Medical Technologies, SoundPipe, Spartan Micro, SpectraWAVE, Surmodics, Terumo, the Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf Medical, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, W.L. Gore, and Xeltis; has received consulting honoraria from Abbott Vascular, Boston Scientific, CeloNova, OrbusNeich, Terumo, W.L. Gore, Edwards Lifesciences, Cook Medical, CSI, ReCor Medical, SinoMedical Sciences Technology, Surmodics, and Bard BD; and is a scientific advisory board member of Medtronic and Xeltis. The other authors have no conflicts of interest to declare.
Figures



References
-
- Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55. - PubMed
-
- Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, East C, Lee DP, Ma A, Ewen S, Cohen DL, Wilensky R, Devireddy CM, Lea J, Schmid A, Weil J, Agdirlioglu T, Reedus D, Jefferson BK, Reyes D, D'Souza R, Sharp ASP, Sharif F, Fahy M, DeBruin V, Cohen SA, Brar S, Townsend RR SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51. - PubMed
-
- Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

