Radiation-induced liver disease: beyond DNA damage
- PMID: 36214587
- PMCID: PMC9928481
- DOI: 10.1080/15384101.2022.2131163
Radiation-induced liver disease: beyond DNA damage
Abstract
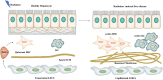
Radiation-induced liver disease (RILD), also known as radiation hepatitis, is a serious side effect of radiotherapy (RT) for hepatocellular carcinoma. The therapeutic dose of RT can damage normal liver tissue, and the toxicity that accumulates around the irradiated liver tissue is related to numerous physiological and pathological processes. RILD may restrict treatment use or eventually deteriorate into liver fibrosis. However, the research on the mechanism of radiation-induced liver injury has seen little progress compared with that on radiation injury in other tissues, and no targeted clinical pharmacological treatment for RILD exists. The DNA damage response caused by ionizing radiation plays an important role in the pathogenesis and development of RILD. Therefore, in this review, we systematically summarize the molecular and cellular mechanisms involved in RILD. Such an analysis is essential for preventing the occurrence and development of RILD and further exploring the potential treatment of this disease.
Keywords: DNA damage; Radiation-induced liver injury; ionizing radiation; molecular and cellular mechanism.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Fan X, Shan S, Wu P, et al. Irradiated and CCl 4 -treated bone marrow-derived liver macrophages exhibit different gene expression patterns and phenotypes. Scand J Immunol. 2020;92(5):e12916. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
