Treatment of Cushing Disease With Pituitary-Targeting Seliciclib
- PMID: 36214832
- PMCID: PMC10210614
- DOI: 10.1210/clinem/dgac588
Treatment of Cushing Disease With Pituitary-Targeting Seliciclib
Abstract
Context: Preclinical studies show seliciclib (R-roscovitine) suppresses neoplastic corticotroph proliferation and pituitary adrenocorticotrophic hormone (ACTH) production.
Objective: To evaluate seliciclib as an effective pituitary-targeting treatment for patients with Cushing disease (CD).
Methods: Two prospective, open-label, phase 2 trials, conducted at a tertiary referral pituitary center, included adult patients with de novo, persistent, or recurrent CD who received oral seliciclib 400 mg twice daily for 4 consecutive days each week for 4 weeks. The primary endpoint in the proof-of-concept single-center study was normalization of 24-hour urinary free cortisol (UFC; ≤ 50 µg/24 hours) at study end; in the pilot multicenter study, primary endpoint was UFC normalization or ≥ 50% reduction in UFC from baseline to study end.
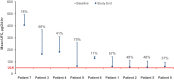
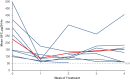
Results: Sixteen patients were consented and 9 were treated. Mean UFC decreased by 42%, from 226.4 ± 140.3 µg/24 hours at baseline to 131.3 ± 114.3 µg/24 hours by study end. Longitudinal model showed significant UFC reductions from baseline to each treatment week. Three patients achieved ≥ 50% UFC reduction (range, 55%-75%), and 2 patients exhibited 48% reduction; none achieved UFC normalization. Plasma ACTH decreased by 19% (P = 0.01) in patients who achieved ≥ 48% UFC reduction. Three patients developed grade ≤ 2 elevated liver enzymes, anemia, and/or elevated creatinine, which resolved with dose interruption/reduction. Two patients developed grade 4 liver-related serious adverse events that resolved within 4 weeks of seliciclib discontinuation.
Conclusion: Seliciclib may directly target pituitary corticotrophs in CD and reverse hypercortisolism. Potential liver toxicity of seliciclib resolves with treatment withdrawal. The lowest effective dose requires further determination.
Keywords: Cushing disease; adrenocorticotrophic hormone; cortisol; pituitary adenoma.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
New Hope for a Tumor-Directed Therapy for Cushing Disease.J Clin Endocrinol Metab. 2023 Feb 15;108(3):e34-e35. doi: 10.1210/clinem/dgac666. J Clin Endocrinol Metab. 2023. PMID: 36378569 No abstract available.
References
-
- Giuffrida G, Crisafulli S, Ferrau F, et al. . Global Cushing's disease epidemiology: a systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2022;45(6):1235–1246. - PubMed
-
- Cardinal T, Zada G, Carmichael JD. The role of reoperation after recurrence of Cushing's disease. Best Pract Res Clin Endocrinol Metab. 2021;35(2):101489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

