Association Between Alberta Stroke Program Early Computed Tomography Score and Efficacy and Safety Outcomes With Endovascular Therapy in Patients With Stroke From Large-Vessel Occlusion: A Secondary Analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT)
- PMID: 36215044
- PMCID: PMC9552045
- DOI: 10.1001/jamaneurol.2022.3285
Association Between Alberta Stroke Program Early Computed Tomography Score and Efficacy and Safety Outcomes With Endovascular Therapy in Patients With Stroke From Large-Vessel Occlusion: A Secondary Analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT)
Erratum in
-
Error in Table.JAMA Neurol. 2023 Feb 1;80(2):216. doi: 10.1001/jamaneurol.2022.4331. JAMA Neurol. 2023. PMID: 36508207 Free PMC article. No abstract available.
Abstract
Importance: Endovascular therapy (EVT) has been found to reduce functional disability in patients with acute stroke due to large-vessel occlusion. However, the extent of the ischemic region, measured using Alberta Stroke Program Early Computed Tomography Scores, may limit the efficacy of EVT.
Objective: To compare the efficacy and safety of EVT according to ASPECTS 3 or less vs 4 to 5.
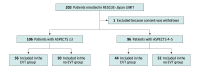
Design, setting, and participants: The Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism-Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT) was an open-label randomized clinical trial conducted from November 2018 to December 2021 at 45 stroke centers across Japan. The trial enrolled adult patients with acute ischemic stroke with a large ischemic region, defined as ASPECTS 3 to 5 primarily determined by magnetic resonance imaging, with occlusion site at the internal carotid artery or middle cerebral artery segment 1. Among 203 enrolled patients, 1 withdrew consent and 202 were included in the original trial and secondary analysis. This secondary analysis was conducted in April 2022.
Interventions: Patients were randomly assigned to EVT with medical therapy or medical therapy alone.
Main outcomes and measures: Modified Rankin Scale (mRS) score at 90 days and symptomatic and any intracranial hemorrhage within 48 hours.
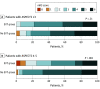
Results: Among 202 patients, 106 (52%) had ASPECTS 3 or less (mean [SD] age, 76.7 [9.6] years; 54 female individuals [50.9%]) and 96 had ASPECTS 4 to 5 (mean [SD] age, 75.6 [10.6] years; 36 female individuals [37.5%]). Of patients with ASPECTS 3 or less, 12 (21.4%) in the EVT group and 9 (18.0%) in the no EVT group had an mRS score of 0 to 3 (odds ratio [OR], 1.24; 95% CI, 0.47-3.26). Of patients with ASPECTS 4 to 5, 19 patients (43.2%) in the EVT group and 4 (7.7%) in the no EVT group had an mRS score of 0 to 3 at 90 days (OR, 9.12; 95% CI, 2.80-29.70; interaction P = .01). The ordinal shift across the range of mRS scores toward a better outcome was not significant in those with ASPECTS or 3 or less (common OR, 1.56; 95% CI, 0.79-3.10) but was significant in those with ASPECTS 4 to 5 (common OR, 4.48; 95% CI, 2.07-9.71; interaction P = .046). The risk of intracranial hemorrhage was significantly increased in patients with ASPECTS 3 or less when EVT was conducted (OR, 4.14; 95% CI, 1.84-9.32) and nonsignificantly increased in those with ASPECTS 4 to 5 (OR, 2.05; 95% CI, 0.89-4.73; interaction P = .24).
Conclusions and relevance: In this study, EVT was associated with improved 90-day functional outcomes in patients with acute large vessel occlusive stroke and ASPECTS was 4 to 5 but not in those with ASPECTS 3 or less.
Trial registration: ClinicalTrials.gov Identifier: NCT03702413.
Conflict of interest statement
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211 - DOI - PubMed
-
- Yamagami H, Hayakawa M, Inoue M, et al. ; JSS/JNS/JSNET Joint Guideline Authoring Committee . Guidelines for mechanical thrombectomy in Japan, the fourth edition, march 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021;61(3):163-192. doi:10.2176/nmc.nmc.st.2020-0357 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

