Pathological Roles for Endothelial Colony-Forming Cells in Neonatal and Adult Lung Disease
- PMID: 36215049
- PMCID: PMC9817912
- DOI: 10.1165/rcmb.2022-0318PS
Pathological Roles for Endothelial Colony-Forming Cells in Neonatal and Adult Lung Disease
Abstract
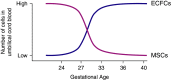
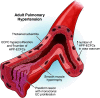
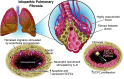
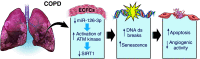
Endothelial colony-forming cells (ECFCs) are vascular resident and circulating endothelial cell subtypes with potent angiogenic capacity, a hierarchy of single-cell clonogenic potentials, and the ability to participate in de novo blood vessel formation and endothelial repair. Existing literature regarding ECFCs in neonatal and adult pulmonary diseases is confounded by the study of ambiguously defined "endothelial progenitor cells," which are often not true ECFCs. This review contrasts adult and fetal ECFCs, discusses the effect of prematurity on ECFCs, and examines their different pathological roles in neonatal and adult pulmonary diseases, such as bronchopulmonary dysplasia, congenital diaphragmatic hernia, pulmonary artery hypertension, pulmonary fibrosis, and chronic obstructive pulmonary disease. Therapeutic potential is also discussed in light of available preclinical data.
Keywords: ECFCs; bronchopulmonary dysplasia; congenital diaphragmatic hernia; pulmonary artery hypertension; pulmonary fibrosis.
Figures


References
-
- Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation . 2005;112:1618–1627. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

