Sleep and wake in a model of the thalamocortical system with Martinotti cells
- PMID: 36215116
- PMCID: PMC10083195
- DOI: 10.1111/ejn.15836
Sleep and wake in a model of the thalamocortical system with Martinotti cells
Abstract
The mechanisms leading to the alternation between active (UP) and silent (DOWN) states during sleep slow waves (SWs) remain poorly understood. Previous models have explained the transition to the DOWN state by a progressive failure of excitation because of the build-up of adaptation currents or synaptic depression. However, these models are at odds with recent studies suggesting a role for presynaptic inhibition by Martinotti cells (MaCs) in generating SWs. Here, we update a classical large-scale model of sleep SWs to include MaCs and propose a different mechanism for the generation of SWs. In the wake mode, the network exhibits irregular and selective activity with low firing rates (FRs). Following an increase in the strength of background inputs and a modulation of synaptic strength and potassium leak potential mimicking the reduced effect of acetylcholine during sleep, the network enters a sleep-like regime in which local increases of network activity trigger bursts of MaC activity, resulting in strong disfacilitation of the local network via presynaptic GABAB1a -type inhibition. This model replicates findings on slow wave activity (SWA) during sleep that challenge previous models, including low and skewed FRs that are comparable between the wake and sleep modes, higher synchrony of transitions to DOWN states than to UP states, the possibility of triggering SWs by optogenetic stimulation of MaCs, and the local dependence of SWA on synaptic strength. Overall, this work points to a role for presynaptic inhibition by MaCs in the generation of DOWN states during sleep.
Keywords: Martinotti cells; computational model; large-scale simulation; sleep; slow wave; somatostatin-positive cells; visual cortex; wake.
© 2022 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Figures
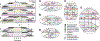





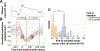


References
-
- Steriade M, Timofeev I & Grenier F Natural Waking and Sleep States: A View From Inside Neocortical Neurons. J. Neurophysiol. 85, 1969–1985 (2001). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

