ARRB2 (β-Arrestin-2) Deficiency Alters Fluid Homeostasis and Blood Pressure Regulation
- PMID: 36215165
- PMCID: PMC9669141
- DOI: 10.1161/HYPERTENSIONAHA.122.19863
ARRB2 (β-Arrestin-2) Deficiency Alters Fluid Homeostasis and Blood Pressure Regulation
Abstract
Background: GPCRs (G protein-coupled receptors) are implicated in blood pressure (BP) and fluid intake regulation. There is a developing concept that these effects are mediated by both canonical G protein signaling and noncanonical β-arrestin mediated signaling, but the contributions of each remain largely unexplored. Here, we hypothesized that β-arrestin contributes to fluid homeostasis and blood pressure (BP) regulation in deoxycorticosterone acetate (DOCA) salt hypertension, a prototypical model of salt-sensitive hypertension.
Methods: Global β-arrestin1 (Arrb1) and β-arrestin2 (Arrb2) knockout mice were employed to evaluate drinking behavior, and BP was evaluated in Arrb2-knockout mice. Age- and sex-matched C57BL/6 mice served as controls. We measured intake of water and different sodium chloride solutions and BP employing a 2-bottle choice paradigm with and without DOCA.
Results: Without DOCA (baseline), Arrb2-knockout mice exhibited a significant elevation in saline intake with no change in water intake. With DOCA treatment, Arrb2-knockout mice exhibited a significant increase in both saline and water intake. Although Arrb2-knockout mice exhibited hypernatremia at baseline conditions, we did not find significant changes in total body sodium stores or sodium palatability. In a separate cohort, BP was measured via telemetry in Arrb2-knockout and C57BL/6 mice with and without DOCA. Arrb2-knockout did not exhibit significant differences in BP before DOCA treatment when provided water alone, or when provided a choice of water and saline. However, Arrb2-knockout exhibited an increased pressor response to DOCA-salt.
Conclusions: These findings suggest that in salt-sensitive hypertension, ARRB2, but not ARRB1 (β-arrestin 1), might counterbalance the canonical signaling of GPCRs.
Keywords: angiotensin; arrestin; blood pressure; homeostasis; hypertension.
Conflict of interest statement
Conflicts of interest
CDS was a member of a Scientific Advisory Board for Ionis Pharmaceuticals in 2020. His contributions to that board are unrelated to the content of this manuscript. There are no other conflicts of interest.
Figures

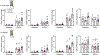
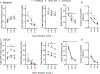

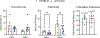

References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. - PubMed
-
- Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public E, Publications Committee of the Council on H, Council on C, Stroke N, Council on Clinical C, Council on G, Precision M, Council on Peripheral Vascular D, Council on Quality of C, Outcomes R and Stroke C. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53–e90. - PMC - PubMed
-
- Hering D and Schlaich M. The Role of Central Nervous System Mechanisms in Resistant Hypertension. Curr Hypertens Rep. 2015;17:58. - PubMed
-
- He FJ and MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

