Randomized, placebo controlled phase I trial of the safety, pharmacokinetics, pharmacodynamics and acceptability of a 90 day tenofovir plus levonorgestrel vaginal ring used continuously or cyclically in women: The CONRAD 138 study
- PMID: 36215267
- PMCID: PMC9550080
- DOI: 10.1371/journal.pone.0275794
Randomized, placebo controlled phase I trial of the safety, pharmacokinetics, pharmacodynamics and acceptability of a 90 day tenofovir plus levonorgestrel vaginal ring used continuously or cyclically in women: The CONRAD 138 study
Abstract
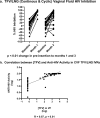
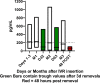
Multipurpose prevention technologies (MPTs), which prevent sexually transmitted infection(s) and unintended pregnancy, are highly desirable to women. In this randomized, placebo-controlled, phase I study, women used a placebo or tenofovir (TFV) and levonorgestrel (LNG) intravaginal ring (IVR), either continuously or cyclically (three, 28-day cycles with a 3 day interruption in between each cycle), for 90 days. Sixty-eight women were screened; 47 were randomized to 4 arms: TFV/LNG or placebo IVRs used continuously or cyclically (4:4:1:1). Safety was assessed by adverse events and changes from baseline in mucosal histology and immune mediators. TFV concentrations were evaluated in multiple compartments. LNG concentration was determined in serum. Modeled TFV pharmacodynamic antiviral activity was evaluated in vaginal and rectal fluids and cervicovaginal tissue ex vivo. LNG pharmacodynamics was assessed with cervical mucus quality and anovulation. All IVRs were safe with no serious adverse events nor significant changes in genital tract histology, immune cell density or secreted soluble proteins from baseline. Median vaginal fluid TFV concentrations were >500 ng/mg throughout 90d. TFV-diphosphate tissue concentrations exceeded 1,000 fmol/mg within 72hrs of IVR insertion. Mean serum LNG concentrations exceeded 200 pg/mL within 2h of TFV/LNG use, decreasing quickly after IVR removal. Vaginal fluid of women using TFV-containing IVRs had significantly greater inhibitory activity (87-98% versus 10% at baseline; p<0.01) against HIV replication in vitro. There was a >10-fold reduction in HIV p24 antigen production from ectocervical tissues after TFV/LNG exposure. TFV/LNG IVR users had significantly higher rates of anovulation, lower Insler scores and poorer/abnormal cervical mucus sperm penetration. Most TFV/LNG IVR users reported no change in menstrual cycles or fewer days of and/or lighter bleeding. All IVRs were safe. Active rings delivered high TFV concentrations locally. LNG caused changes in cervical mucus, sperm penetration, and ovulation compatible with contraceptive efficacy. Trial registration: ClinicalTrials.gov #NCT03279120.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- UNAIDS. Prevention Gap Report. http://wwwunaidsorg/sites/default/files/media_asset/2016-prevention-gap-... [Internet]. 2016.
-
- Kott A. Rates of Unintended Pregnancy Remain High In Developing Regions. International Perspectives on Sexual and Reproductive Health. 2011;37(1).
-
- Macaluso M, Blackwell R, Jamieson DJ, Kulczycki A, Chen MP, Akers R, et al.. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. Am J Epidemiol. 2007;166(1):88–96. Epub 2007/04/11. doi: 10.1093/aje/kwm046 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

