A genome-wide screen identifies SCAI as a modulator of the UV-induced replicative stress response
- PMID: 36215310
- PMCID: PMC9584372
- DOI: 10.1371/journal.pbio.3001543
A genome-wide screen identifies SCAI as a modulator of the UV-induced replicative stress response
Abstract
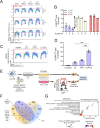
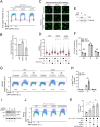
Helix-destabilizing DNA lesions induced by environmental mutagens such as UV light cause genomic instability by strongly blocking the progression of DNA replication forks (RFs). At blocked RF, single-stranded DNA (ssDNA) accumulates and is rapidly bound by Replication Protein A (RPA) complexes. Such stretches of RPA-ssDNA constitute platforms for recruitment/activation of critical factors that promote DNA synthesis restart. However, during periods of severe replicative stress, RPA availability may become limiting due to inordinate sequestration of this multifunctional complex on ssDNA, thereby negatively impacting multiple vital RPA-dependent processes. Here, we performed a genome-wide screen to identify factors that restrict the accumulation of RPA-ssDNA during UV-induced replicative stress. While this approach revealed some expected "hits" acting in pathways such as nucleotide excision repair, translesion DNA synthesis, and the intra-S phase checkpoint, it also identified SCAI, whose role in the replicative stress response was previously unappreciated. Upon UV exposure, SCAI knock-down caused elevated accumulation of RPA-ssDNA during S phase, accompanied by reduced cell survival and compromised RF progression. These effects were independent of the previously reported role of SCAI in 53BP1-dependent DNA double-strand break repair. We also found that SCAI is recruited to UV-damaged chromatin and that its depletion promotes nascent DNA degradation at stalled RF. Finally, we (i) provide evidence that EXO1 is the major nuclease underlying ssDNA formation and DNA replication defects in SCAI knockout cells and, consistent with this, (ii) demonstrate that SCAI inhibits EXO1 activity on a ssDNA gap in vitro. Taken together, our data establish SCAI as a novel regulator of the UV-induced replicative stress response in human cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

